doi: 10.56294/evk202266
ORIGINAL
Quantitative study of the variable pollutant load in hospital wastewater from the Imbanaco Clinic in the city of Cali
Estudio cuantitativo de la carga contaminante variante de las aguas residuales hospitalarias de la Clínica Imbanaco de la ciudad de Cali
Sara Juliana Jaramillo Arvilla1, Julián Diel Urresta Aragón1, Natali Lorena Mena Guerrero1, Carla Stephanny Cárdenas Bustos1
1Universidad de Pamplona, Facultad de Ingenierías y Arquitectura, Programa de Ingeniería Química, Norte de Santander. Pamplona, Colombia.
Cite as: Jaramillo Arvilla SJ, Urresta Aragón JD, Mena Guerrero NL, Cárdenas Bustos CS. Quantitative study of the variable pollutant load in hospital wastewater from the Imbanaco Clinic in the city of Cali. eVitroKhem. 2022; 1:66. https://doi.org/10.56294/evk202266
Submitted: 01-02-2021 Revised: 14-01-2022 Accepted: 19-07-2022 Published: 20-07-2022
Editor: Prof.
Dr. Javier Gonzalez-Argote ![]()
ABSTRACT
Water contamination by emerging contaminants due to human activities has become one of the most critical difficulties in recent years. Within this problem of wastewater, we find hospital wastewater, catalogued as an important source of environmental risk due to the presence of metabolites and emerging micro contaminants. This wastewater includes laundry, kitchen, cleaning and diagnostic services, as well as care, laboratory, research and diagnostic activities. It is a challenge for health service providers to address this problem and comply with current environmental regulations. There are different processes, including advanced oxidation processes, in this case by ozonation. However, it is important to know the pollutant nature of each effluent that is why the purpose of this research was to identify the organic load contributed by the liquid waste from the headquarters of the Imbanaco Medical Center located in the city of Cali, Valle del Cauca, the experimental design evaluated allows concluding that the cleaning and sterilization supplies used in the Imbanaco Medical Center are highly recalcitrant and exceed the maximum permissible limits established by resolution 0631 of 2015.
Keywords: Hospital Wastewater; Physicochemical Parameters; Oxidation Process; Micro Pollutants.
RESUMEN
La contaminación del agua por contaminantes emergentes debido a actividades humanas se ha convertido en una de las dificultades más críticas en los últimos años. Dentro de esta problemática de aguas residuales, se encuentran las de tipo hospitalario, catalogadas fuente importante de riesgo ambiental por la presencia de metabolitos y micro contaminantes emergentes. Estas aguas servidas comprenden los servicios de lavandería, cocina, limpieza, diagnóstico, así como las actividades de atención, los laboratorios, investigación, y diagnóstico. Es un reto para las entidades prestadoras de servicios de salud atender a esta problemática y dar cumplimiento a las normas ambientales vigentes. Se encuentran diferentes procesos entre ellos los procesos de oxidación avanzada, en este caso por ozonización. Sin embargo, es importante conocer la naturaleza contaminante de cada efluente es por eso que el propósito de esta investigación fue identificar la carga orgánica que aportan los desechos líquidos de las sedes del Centro Médico Imbanaco ubicado en la ciudad de Cali, Valle del Cauca, el diseño experimental evaluado permite concluir que los insumos de limpieza y esterilización utilizados en el Centro Médico Imbanaco son altamente recalcitrantes y superan los límites máximos permisibles establecidos por la resolución 0631 del 2015.
Palabras clave: Aguas Residuales Hospitalarias; Parámetros Fisicoquímicos; Proceso de Oxidación; Micro Contaminantes.
INTRODUCTION
The Imbanaco Medical Center (CMI) in the city of Cali has been recognized on multiple occasions for its advances in hospital safety, receiving on five occasions the "National Safe Hospital Award" granted by the Colombian Association of Hospitals and Clinics (ACHC).(1,2,3,4,5) This recognition highlights the continuous improvements in the quality of care and institutional management.(6,7,8,9,10) As part of its commitment to sustainable development, one of WCC's priorities is proper environmental management, particularly the treatment of wastewater generated by its health care services.(11,12,13,14,15)
Hospital wastewater represents a major environmental challenge due to the presence of emerging contaminants and recalcitrant compounds such as pharmaceuticals, radionuclides, solvents and disinfectants, which are generated in various clinical, laboratory and research activities.(1,16,17,18,19,20) Inadequate management of these effluents can generate negative impacts on public health and ecological balance, including disease outbreaks, contamination of water bodies and accumulation of toxic substances.(21,22,23,24,25) Due to their complexity, these wastes are not effectively treated by conventional municipal treatment systems, which requires the implementation of advanced and economically viable technologies.(26,27,28,29,30)
In this context, the present research was developed in collaboration with the company Water Treatment Colombia S.A.S., a spin-off of the Universidad del Valle specialized in cleaner production processes. The objective of the study was to characterize the pollutant load of hospital wastewater from the Imbanaco Medical Center and to evaluate the application of advanced oxidation techniques as an alternative for its treatment. For this purpose, sampling and analysis of physicochemical parameters were carried out to determine the effectiveness of these technologies in the mineralization of pollutants present in the effluents.
The results of this study seek to contribute to technical knowledge in the management of hospital wastewater and offer tools for decision making in the design of sustainable treatment systems in the health sector.
What type of pollutant load is present in the hospital wastewater of the Imbanaco Medical Center and how effective is the application of advanced oxidation techniques for its treatment?
Objective
To study the nature of the variant pollutant load of hospital wastewater from the Imbanaco clinic in the city of Cali.
METHOD
In order to analyze the behavior of physicochemical parameters according to resolution 0631 of 2015, the following methodology is established based on the standard methods approved by the scientific community for measurements in wastewater.
Sampling
Sampling for control and monitoring
A sample collector, a bucket, a container for sample storage, and an icopor cooler to refrigerate the samples were used for sample collection. The sample storage container depends on the measurement protocol established in the standard methods. The samples were classified into point and composite samples, a point sample is one that is collected at a specific point of a site during a short period of time (usually minutes or seconds) and a composite sample is one that is made up of a mixture of point samples, collected at different points simultaneously or as synchronized as possible. The sample is finally preserved with sulfuric acid or hydrochloric acid and the sample volume was 500 mL.
Main site
The main office is located at Carrera 38 Bis No. 5B2 - 04, Cali, Valle del Cauca. It provides services such as radiotherapy, nuclear medicine, emergency, diagnostic imaging, outpatient, cardiology, angiography, endoscopy, resonance, ICU, surgery, chemotherapy, comprehensive care of women, pathology laboratory, clinical laboratory, sterilization center, casino, hospitalization, telemetry and administrative areas. The main headquarters has 3 effluent outlets (wastewater boxes), these outlets were monitored on September 24 by the HIDROAMBIENTAL laboratory certified before the Institute of Hydrology, Meteorology and Environmental Studies (IDEAM), where in the first instance point samples were taken every 20 minutes for 12 hours, in order to directly measure pH, temperature and flow rate and manage to form a composite sample at the end of the sampling day, in order to analyze the physicochemical parameters described in resolution 0631 of 2015. Figure 1 shows the monitoring points of the main site with their address, coordinates and specifications of each point. In addition, photos of these monitored points are presented in figures 2-3.

Figure 1. Monitoring points of the Imbanaco Medical Center main headquarters

Figure 2. Monitoring point 1 of the main headquarters

Figure 3. Monitoring point 2 of the main site

Figure 4. Monitoring point 3 of the main office
Tower A and B
Tower A and B of the Imbanaco de Cali S.A. Medical Center are located at Carrera 38 A # 5 A - 100. For Tower A, the gauging and sampling day was carried out during 6 hours of monitoring, at five points that receive domestic and process effluents. Tower A provides the following services: Diagnostic Imaging, Radiosurgery (GammaKnife), Ophthalmology, Cardiac Rehabilitation, cafeteria and medical offices. Figure 5 shows the monitoring points of Tower A with their addresses, coordinates and specifications of each point. In addition, photos of these monitored points are shown in figures 6-20.

Figure 5. Monitoring points of Tower A of Imbanaco Medical Center

Figure 6. Tower A monitoring point 1

Figure 7. Tower A monitoring point 2

Figure 8. Tower A monitoring point 3

Figure 9. Tower A monitoring point 4

Figure 10. Tower A monitoring point 5
And for Tower B, the gauging and sampling day was carried out during 6 hours of monitoring at the only point that receives domestic and process effluents. Tower B provides clinical laboratory services, vaccination, Reproductive Medicine Unit and medical offices.
The general effluent point corresponds to the chamber located on the platform of Tower B, on Carrera 38 A, which receives all the effluents from this tower, including the clinical laboratory. The dimensions of the chamber are 88 cm * 88 cm on each side and a depth of 90 cm, which receives a pipe of 5,98 in. Table 1 shows the coordinates of the monitoring point of Tower B and figure 11 shows the photo of the point.
|
Table 1. Geodetic coordinates of the Tower B monitoring point |
|
|
Coordinate Latitude |
s geodetic coordinates Longitude |
|
North 03°25'26,6'' N |
West 76°32'39,2'' W |

Figure 11. General monitoring point of Tower B
Rehabilitation and physical medicine unit
The rehabilitation and physical medicine unit is located at Calle 5B No. 38A-24 and provides physiatry, physical rehabilitation, muscle strengthening, physical conditioning and manual lymphatic drainage services.
The site does not process analyses; the wastewater generated comes from cleaning the facilities, toilets, and hand-washing water. In order to obtain representative results of the operation of the Rehabilitation and Physical Medicine Unit, the gauging and sampling day was carried out for 6 hours every 20 minutes, composing a sample for analysis.
The gauging and sampling was carried out at the point corresponding to the domiciliary chamber located at the main entrance. Two 5,98 in. pipes from the bathrooms and toilets reach this chamber. Table 2 shows the coordinates of the monitoring point of the Rehabilitation and Physical Medicine Unit and figure 12 shows the photo of the point.
|
Table 2. Geodetic coordinates of the monitoring point of the Physical Medicine and Rehabilitation Unit |
|
|
Coordinate Latitude |
s geodesic coordinates Longitude |
|
North 03°25'28,0'' N |
West 76°32'56,2'' W |

Figure 12. General monitoring point of the rehabilitation and physical medicine unit
Estimation of the nature of the pollutant load
For the monitoring of physical and chemical parameters of hospital wastewater, samples were taken at the entry and exit points during 8 months. Table 3 shows the parameters that were evaluated, the equipment or technique used for the analysis, the frequency with which the measurements were taken and the points sampled.
|
Table 3. Site, frequency and physicochemical variables to be measured |
||||
|
Parameters |
Unit |
Equipment |
Frequency of measurement |
Sampling point |
|
Flow rate |
L/s |
Capacity |
1/day |
Inlet and outlet |
|
pH |
pH units |
|
4/day |
Input, Output, Intermediate |
|
Temperature |
°C |
|
4/day |
Inlet, Outlet, Intermediate |
|
Dissolved Oxygen (DO) |
mg/L |
Multiparameter Water Quality Probe HI- 98194 Hanna Instruments |
4/day |
Input, Output, Intermediate |
|
Electrical Conductivity (EC) |
mS/cm |
4/day |
Input, Output, Intermediate |
|
|
Oxidation Reduction Potential (ORP) |
mV |
4/day |
Input, Output, Intermediate |
|
|
Ammonium (NH4+) |
mg/L |
|
3/week; 2/day |
Input, Output, Intermediate |
|
Nitrate (NO3) |
mg/L |
|
3/week; 2/day |
Input, Output, Intermediate |
|
Alkalinity |
mg/L |
HI775 Checker - Hanna Instruments |
3/week; 2/day |
Input, Output |
|
Phosphates (P-PO4-3) |
mg/L |
HI717 Checker - Hanna Instruments |
3/week; 2/day |
Input, Output |
|
Laboratory Parameters |
Unit |
Standard Methods (2017) |
Frequency of measurement |
Sampling point |
|
Total Nitrogen Kjeldahl (NTK) |
mg/L |
4500 Norg- B, C |
1/week |
Input, Output |
|
Chemical Oxygen Demand (COD) |
mg/L |
5220-D |
1/week |
Input, Output |
|
Biochemical Oxygen Demand Oxygen Demand (BOD5) |
mg/L |
5210-D |
1/week |
Input, Output |
|
Dissolved Organic Carbon (DOC) |
mg/L |
5310-B |
1/week |
Input, Output |
|
Total Suspended Total Suspended Solids (TSS) |
mg/L |
2540-D |
1/week |
Input, Output |
To determine the nature of the molecules present in the drugs, a Gas Chromatography coupled to Mass Spectrometry system was used with the conditions mentioned in table 4 and, according to the NIST Research Library, the percentages of existence were estimated.
|
Table 4. Conditions of the Gas Chromatography coupled to Mass Spectrometry (GCMS) system |
|
|
Programming of the oven temperature |
Initial temperature of 40°C at an initial time of 2 min and ends with a temperature of 280°C after 20 min. |
|
Gas flow rate |
13,3°C/min |
|
Injection mode |
Split (1,2 µ L) |
|
Column |
SH-Rxi-5Sil Ms (30 m long, 0,25 mm overlay) |
|
Ionization mode |
SEI |
|
Detector |
15 eV and 70 eV |
|
Analysis time (min) |
3,0 - 75,0 |
|
Transfer line temperature Transfer line temperature |
230°C |
|
Column pressure |
46kPa |
|
Column flow rate |
1mL/min |
Advanced Oxidation Process
Ozonization System
Ozone
The ozone oxidation process is a highly efficient wastewater treatment that uses variable injection of the chemical compound ozone to eliminate organic and inorganic contaminants.
Ozone is more efficient than chlorine and has the added advantage of producing no chemical waste, since ozone degradation is complete because ozone reacts and converts to O2.
Under normal environmental conditions, oxygen is a molecule consisting of two atoms. These two atoms are joined by a double bond (O=O). If energy is supplied to this molecule, one of these bonds is broken. An additional oxygen atom can now bond. This produces a molecule consisting of three oxygen atoms: ozone, as seen in figure 13.

Figure 13. Schematic of ozone production
Ozone is generated by the reaction of an oxygen molecule with an oxygen atom using the electrical discharge principle. In this process, a gas containing oxygen (usually air or pure oxygen) is passed through an electric field between two electrodes. It must be ensured beforehand that the gas is dry and free of dust particles. The oxygen is converted to ozone in the electric field. The resulting ozone gas stream is transported directly to where it is needed (e.g. to the mixing equipment to dissolve it in the wastewater).
The ozone molecule decomposes again after a short time. This produces oxygen and heat. This short shelf life prevents ozone from being produced in high concentrations and stored. Ozone must be produced on site. In its concentrated form, ozone is a colorless gas, which is about 1,5 times heavier than air. Therefore, if ozone escapes, it can accumulate at ground level. This odor can still be noticed at a concentration of 1: 500 000. The odor threshold for ozone is about 0,04 mg/m3.
Ozone is technically the strongest oxidizing agent compared to chlorine dioxide, hydrogen peroxide, peridroxyl radical, hypochlorous acid and chlorine. This property is the fundamental reason for the use of ozone in the treatment and disinfection of drinking water, process water and wastewater. Undesirable substances are oxidized into easily removable or biodegradable substances. The main advantage of ozone is that it decomposes back into oxygen after use, which in any case is already present in the water.
Treatment system
Figure 14 shows the scheme and components of the process, which is explained as follows: Air extracted from the environment is compressed to 30 psig, then passes through a drying system to remove water; The dry, compressed air enters the oxygen generation system, here a series of filters adsorbs nitrogen from the air and produces an oxygen-enriched gas stream. Ozone is produced as described above inside a tube containing the corona discharge cell, then injected into the water by bubble diffusers in the contact tank. Cooling water (18 to 20°C) from a chiller is used to dissipate the heat generated in the cell.

Figure 14. Ozonation process for hospital wastewater treatment
Compressor
The compressor system is used to feed pressurized, oil-free air to the ozone generation system. Table 5 shows the specifications of the air compressor.
|
Table 5. Air compressor specifications |
|
|
Model |
Mzb 1500G |
|
Power |
1500 W |
|
Dimensions |
550 x 600 x 200 (millimeter) |
|
Power supply |
110 V / 60 Hz |
|
Current |
13 amps max. |
Ozone generator system
Figure 15 shows the elements of the ozone tube. The element consists of an outer grounded stainless steel tube, a high voltage electrode and a dielectric. Gas passes through the gap between the high voltage electrode, the dielectric and the outer electrode where ozone is formed by an electrical discharge.
The electrical discharge is enabled by a mid-range frequency alternating high voltage signal installed between the high voltage electrode and the outer tube, and causes a proportion of the oxygen to be converted to ozone. The heat generated during discharge is released through the pipe wall to the cooling water flowing between the outer metal pipe and the outer pipe. This direct cooling provides excellent heat transfer to the cooling water and thus an excellent degree of efficiency of the ozone generating elements.
Table 6 shows the specification of the ozone generating system. As mentioned, the oxygen and ozone generating elements are located inside a stainless steel cabinet, with two inlet ports (compressed air and cooling water) and two outlet ports (ozone outlet and cooling water). Figure 16 shows a photograph of the cabinet.

Figure 15. Elements of the ozone tube
|
Table 6. Specifications of the ozone generator system |
|
|
Model |
WTC40 |
|
Ozone output |
40 g / h |
|
Max. Ozone concentration |
80 ppm |
|
Power |
550 W |
|
Dimensions |
550 x 400 x 1240 (millimeter) |
|
Power supply |
110 V / 60 Hz |
|
Current |
15 Ampere |
|
Gas supply |
Compressed and dry air |

Figure 16. Photograph of the ozone generation system WTC S.A.S
Cooler
The cooling water has the function of dissipating the heat produced during ozone generation in the ozone generator chamber. This heat dissipation is important to keep the ozone gas at a low temperature to maintain optimum ozone output and protect the ozone generator's internal components. The cooling water travels from the 1,0 ton chiller through the heat exchanger and then back to the chiller via a centrifugal pump. The cooling water temperature (between 18 and 22°C) is regulated by an automated system consisting of a thermocouple and an on/off switch. Table 7 shows some specifications of the chiller and its components.
|
Table 7. Cooling system specifications |
|
|
Cooling capacity |
1 ton |
|
Temperature |
15 to 22 ° C |
|
Cooling water flow rate |
1 l / s |
|
Power |
1,5 kW |
|
Dimensions |
550 x 500 x 1200 (millimeter) |
|
Power supply |
220 V / 60 Hz |
|
Current |
15 amps |
Sampling of the ozonation system
Samples were taken punctually at the inlet and outlet of the ozone oxidation system using a volumetric instrument (measuring cylinder).
Improvement of the efficiency of the oxidation process
To demonstrate the improvement of the oxidation process, the technical data sheets of the installed equipment and process diagrams were reviewed. To document the follow-up, a logbook and folders with documents and calculation memories were created. All the results and indicators of the aforementioned activities were supported by milestones established in the process follow-up document Water Treatment Colombia S.A.S. Figure 17 shows a photograph of the ozonation process installed at the Imbanaco Medical Center.

Figure 17. Photograph of the ozonation process installed at Imbanaco Medical Center
The removal technique used is an advanced oxidation technique, with ozone generating equipment, which generates ozone from an electrical discharge with oxygen generated by a 110 V current, and then injected into the treatment tank with a microbubble bubbling with stainless steel diffusers in a contact time of 6 hours, which is necessary for the treatment of the effluent.
RESULTS
Nature of pollutant load
Analysis of cleaning products
The activities related to the study of discharges of the Imbanaco Medical Center (CMI) began by knowing and analyzing the pollutant nature of the cleaning and disinfection supplies in order to know the possible influences on the physicochemical parameters in the punctual discharges of hospital wastewater.
Analyzing the results of the previous year, the importance of periodically monitoring the effluents generated at the Imbanaco Medical Center's headquarters, Tower A, Tower B and the Rehabilitation and Physical Medicine Unit became evident.
The most representative results during the study of the nature of the cleaning and disinfection products are shown below.
· Los products such as PERACETIC is an input that is composed of peracetic acid and hydrogen peroxide, which can represent an important alteration in the parameters of total acidity, total alkalinity, total hardness, COD, BOD5, and fats and oils. In addition, this input, due to its high level of use, is the one that causes a high pH in the grease trap.
· El detergent Gold, Clorin, Hand Quat, and Soap Al 35 cory are disinfection products and could trigger an alteration in the parameters of phenols, total acidity, alkalinity, methylene blue active substances (SAAM), COD and BOD5; therefore, a good use of these products is recommended with the necessary quantities.
· El BH-38 detergent with solvent is a product that contains a combination of detergent and butoxyethanol with sodium dodecyl benzene sulfonate and 2-butoxyethanol and BIOCORY SEC EXTRA-PLUS is a product with quaternary ammonium, these products would present an alteration to the parameters of phenols, total acidity, alkalinity, active substances to methylene blue (SAAM), COD and BOD5. It is important the good use of this product with the necessary quantities and responsibility in the handling of this product.
· Crema Frotex: depending on the composition and uses of this product, it could represent a considerable alteration in the parameters of total acidity, total alkalinity, total hardness, COD, BOD5, and fats and oils. In addition, it is an input that depending on its level of exposure can cause eye and skin irritation, so it is recommended that for handling in high quantities or high exposure to use a minimum of protection such as gloves and safety goggles.
· Jabón Multipurpose Liquid: the composition and use of this input, according to its technical and safety data sheet, would not represent a variation in the parameters, since it is used exclusively for hand washing and this product has a high or high solubility, it is recommended to store it in a dry and cool place, protected from sunlight and excessive heat.
· Biodegradable Cleaner Disinfectant: the composition and use of this product, according to its technical and safety data sheet, represents a considerable change in the parameters of total acidity, total alkalinity, phenols, nitrogen compounds and fats and oils. Also, it is a product that depending on its use it is recommended to use the appropriate safety clothing because it could cause eye and skin irritation, it is recommended to store in a dry and cool place, protected from sunlight and excessive heat.
· Acabado Durabrite: this product according to its composition, technical and safety data sheet could generate modifications in the parameters of phenols, orthophosphates, total phosphorus, COD and BOD5. In addition, depending on its use and exposure, it is recommended to use appropriate safety clothing to avoid any adverse effect, even though it is not considered toxic.
· Stain remover Oxy Fresh-Er: according to the composition and uses given to this product according to its technical and safety data sheet, it could represent a considerable alteration in the parameters of total acidity, total alkalinity, total hardness, and fats and oils. It is also an input that depending on its level of exposure can cause eye and skin irritation, so it is recommended that for handling in high quantities or high exposure to use a minimum of protection such as gloves and safety glasses.
· Universal Industrial Absorbent: the composition and use of this input, according to its technical and safety data sheet, could represent a considerable change in the parameters of phenols, total acidity, alkalinity, methylene blue active substances (SAAM), COD and BOD5. In addition, the main function of this product is to intervene quickly in the event of an accidental spill, absorbing all types of fluids, from water to hydrocarbons.
· Limpia Klin: this product according to its composition, technical and safety data sheet could generate modifications in the parameters of active substances to methylene blue (SAAM), total acidity, total alkalinity, total hardness. It is also an input that depending on the level of exposure can cause eye and skin irritation, so it is recommended that for handling in high quantities or high exposure to use a minimum of protection such as gloves and safety glasses, it is important to know that this product is corrosive to metals.
· Bleach Oxygenated Biodegradable Powder: according to the composition and uses given to this product, according to its technical and safety data sheet, it could represent a considerable alteration in the parameters of total acidity, total alkalinity, total hardness, calcium hardness and oils and fats. It is also an input that depending on its level of exposure can cause eye injuries, is harmful if swallowed and is oxidizing, so it is important to wear appropriate safety clothing.
· Stain remover Supercleaner: this product according to its composition, technical and safety data sheet could generate modifications in the parameters of phenols, total acidity, total alkalinity, nitrates, nitrites, active substances to methylene blue (SAAM). This input is used strictly for cleaning surfaces, so it is important to wear appropriate clothing as it could cause eye or skin irritation and is also corrosive to metals.
· Glass Cleaner: depending on the composition and uses of this product, according to its technical and safety data sheet, it could represent a considerable alteration in the parameters of phenols, total acidity, alkalinity, methylene blue active substances (SAAM), COD and BOD5. In addition, it is an input that depending on its level of exposure can cause eye and skin irritation, so it is recommended that for handling in high quantities or high exposure to use adequate protection.
· Stripper Finish Remover: this product according to its composition, technical and safety data sheet could generate modifications in the parameters of Active Substances to Methylene Blue (SAAM), Total Acidity, Total Alkalinity, Total Hardness and Phenols. It is also an input that depending on the level of exposure can cause eye damage, is harmful if swallowed and is oxidizing, so it is important to wear appropriate safety clothing.
· Bonaire Fabrics & Carpets X 400 Gr Powder: the composition and the use given to this input, according to its technical and safety data sheet, would not represent a variation in the parameters, since its use is exclusively for carpets, this product has a solubility in all portions and temperatures, it is recommended to store in a dry and cool place, protected from sunlight and excessive heat.
· CIDEX® OPA Solution: according to its technical and safety data sheet, it could represent a considerable alteration in the parameters of total acidity, total alkalinity, total hardness, COD and BOD5. In addition, it is an input that depending on its level of exposure can cause symptoms similar to asthma (pain and tightness in the chest and difficulty breathing) and aggravate pre-existing asthma, so it is recommended that for its handling in high quantities or high exposure to use a minimum of protection such as masks and safety glasses.
· CIDEZYME* enzymatic detergent: the composition and use of this input, according to its technical and safety data sheet, could represent a considerable change in the parameters of phenols, total acidity, alkalinity, methylene blue active substances (SAAM), COD and BOD5. This product depending on its use and exposure can cause respiratory tract and skin irritation, serious eye injuries, so it is important to use appropriate safety clothing.
· SURFALKAN SH: this product according to its composition, technical and safety data sheet could generate modifications in the parameters of phenols, total acidity, total alkalinity, total hardness, COD, and BOD5. It is also an input that depending on its level of exposure can cause eye and skin irritation, so it is recommended that for handling in high quantities or high exposure to use a minimum of protection such as gloves and safety goggles.
· DETERGENTE LIQUID: the composition and use of this input, according to its technical and safety data sheet, could represent a considerable change in the parameters of total acidity, alkalinity, methylene blue active substances (SAAM), and fats and oils. It is also an input that depending on its level of exposure can cause eye and skin irritation, so it is recommended that for handling in high quantities or high exposure to use a minimum of protection such as gloves and safety goggles.
· WESCOHEX 270: depending on the composition and use of this product, according to its technical and safety data sheet, it could cause a considerable modification in the parameters of total acidity, alkalinity, methylene blue active substances (SAAM), and fats and oils. Depending on its use and exposure, this product may cause eye and skin irritation, so it is recommended to wear appropriate safety clothing. For its storage it is advisable to keep it in a dry, cool and well ventilated place. This product is considered biodegradable in wastewater treatment.
· PREPODYNE SCRUB: the composition and use of this product, according to its technical and safety data sheet, could cause a considerable change in the parameters of total acidity, alkalinity, methylene blue active substances (SAAM), and fats and oils. It is also an input that depending on its level of exposure can cause eye and skin irritation, so it is recommended that for handling in high quantities or high exposure to use a minimum of protection such as gloves and safety goggles.
· DENTAL CREAM: according to the composition and uses of this product, according to its technical and safety data sheet, it could represent a considerable alteration in the parameters of total acidity, total alkalinity, total hardness, calcium hardness, COD, BOD5, and fats and oils. It is also an input that, depending on the level of exposure, can cause eye and skin irritation, so it is recommended that the amounts used be moderate.
The physicochemical parameters measured for each of the inputs were processed taking into account the dilutions suggested by the company that manages the cleaning outsourcing of the Imbanaco Medical Center (RAPIASEO S.A.S.) previously validated by the infectious diseases committee.
Identification of the nature of organic and inorganic compounds
In order to know the percentage of stock of medicines, composite samples were taken from the Imbanaco Medical Center's sites. They were analyzed by means of the Gas Chromatography technique coupled with Mass Spectroscopy to understand the composition of the effluents, these results are shown in figure 18.


Figure 18. Mass Spectroscopy results
· Oxirano: this is a chemical substance mainly used to manufacture ethylene glycol (a chemical substance used as antifreeze and polyester); therefore, the presence of this compound suggests that air conditioning condensate ducts are reaching the discharges.
· Acetone: product of discharges of blood serum from diabetic patients (confirmed by mass spectroscopy).
· Isobutanol: product of compound discharges associated with disinfection (confirmed by mass spectroscopy).
· Peróxido of hydrogen: product of compound discharges associated with disinfection, it could be considered acetic and peracetic acids from detergents.
Behavior of the physicochemical parameters in each of the sampling points of the Imbanaco clinic in the city of Cali
Taking into account that the entity has to be governed under the provisions of Article 14 of resolution 0631 of 2015, and that Article 16 stipulates the maximum permissible limit values.
for each parameter, a range of values can be observed in figures 23-28, where the variations of the parameters studied are captured, placing the threshold of Article 16 with red color to indicate the urgent need to take corrective measures to bring the indicator below this value, and that progressively the removal of pollutant load can be performed until it is below the limit of Article 14, placed in blue color.
Behavior of biochemical oxygen demand
As can be seen in figure 19 for the BOD5 parameter, from 2015 to 2019, the trend was generally positive for the decrease in the pollutant load measured by this indicator, reaching values not only below those established in Article 16 but also maintaining compliance with Article 14. However, by 2020, a variation of up to 100 % can be observed in some sampling points, producing a non-compliance not only with Article 14 but also with Article 16 in the case of the Rehabilitation and Physical Medicine section, this is triggered by the incorrect use of the dosages and composition of the cleaning supplies. As for 2021, a reduction can be observed with respect to this indicator in the sampling points due to the improvement plan proposed by Water Treatment Colombia S.A.S.

Figure 19. Historical 2015-2021 of the "Biochemical Oxygen Demand" (BOD5) parameter for the different sampling zones
Behavior of chemical oxygen demand
Figure 20 shows a similar behavior but tending to comply with Resolution 0631; however, by 2020 there is an increase again, which means that three of the four monitored points do not comply with Article 16; this is due to the services provided in the monitored zones, For the year 2021 there is still non-compliance with Article 14 to Article 16 in two of the monitored points, this is mainly due to the high concentration and demand of cleaning and disinfection supplies, as for the two points that do comply, it is due to the solutions proposed by WTC S.A.S.

Figure 20. Historical 2015-2021 of the parameter "Chemical Oxygen Demand" (COD) of the different sampling zones
Behavior of settleable solids
In the case of Sedimentable Solids, in figure 21, although most of the samples are in compliance with Article 14, an increasing trend of these values can be observed in the years from 2018 to 2020 and it is suggested to perform a preventive measure in what concerns Tower B, Main Headquarters and the Rehabilitation section, such as the proper maintenance of each monitoring point and the dosage of the oxidizing agent already proposed by Water Treatment Colombia S.A.S, as for Tower A corrective measures of treatments should be performed to reduce in case the solids. As for 2021, it was proposed the continuous dosing of an oxidizing agent by
As for 2021, continuous dosing of an oxidizing agent by WTC S.A.S. was proposed to help mineralize the pollutant load at the monitored points.

Figure 21. Historical 2015-2021 of the parameter "Sedimentable Solids" (SSED) of the different sampling zones
Behavior of total suspended solids
Total Suspended Solids do not show any improvement over the last six annual periods reported. In 2021 only the Rehabilitation and Physical Medicine Unit complies with Article 14 to Article 16, this is due to the fact that in this site the flow of patients is minimal compared to the other sites, therefore the amount of total suspended solids decreases, in the other monitoring points, Article 16 is not complied with (and, therefore, with Article 14), As can be seen in figure 22, this may be related mainly to the waste toilet paper and hand towels that are sent by the people who use the sanitary facilities, so it is proposed for the next annual period to conduct training on the proper disposal of these solid wastes.

Figure 22. Historical 2015-2021 of the "Total Suspended Solids" (TSS) parameter for the different sampling zones
Behavior of fats, oils and grease
Figure 23 shows the behavior of Fats and Oils, in previous years there is a significant increase exceeding the maximum permissible limits, therefore, it does not comply with resolution 0631 of 2015, due to excessive use of cleaning agents in this case detergents, degreasers and in the case of Peracetic, since it is used as a disinfectant for food supplies, on the other hand, the poor design and cleaning of the grease traps in the cafeterias and cafeterias, and with respect to the main headquarters, the kitchen traps do not favor an optimal primary treatment. In 2021, the concentration decreases, which was reflected in the compliance with Article 14 and 16, due to the improvement of the design of the grease traps of each service and the implementation of a treatment with a low dose of oxidant to mineralize the pollutant load.

Figure 23. Historical 2015-2021 of the "Fats and Oils" (G&A) parameter for the different sampling zones
Behavior of Phenols
The quantified pollutant load in Phenols has remained constant and below what is established by Article 14 during the entire period studied, as can be seen in figure 24. However, since 2018 it shows an increase in several of the sections taken into account, this is mainly due to the increase in the use of cleaning and disinfection agents such as BH-38 detergent with solvent, Universal Industrial Absorbent among others, due to the global contingency related to the COVID-19 Pandemic. It is suggested to continue monitoring this parameter to avoid non-compliance with Resolution 0631 of 2015.

Figure 24. Historical 2015-2021 of the parameter "Phenols" (PhOH) of the different sampling zones
Performance of the ozonation system for hospital water treatment: Clinical laboratory and surgery
Efficiency of the installed process
In order to improve the efficiency of the installed process, its performance was evaluated by comparing the reduction percentages of parameters such as Chemical Oxygen Demand and Phenol, monthly during 7 months at the ozonizer inlet and outlet. The ozonation process was carried out for 6 hours and has an efficiency of 98 % for the reduction of the organic load and for the reduction of phenol it has an efficiency of 80 %. The results obtained from the monthly monitoring of the oxidation system are detailed below.
Analysis of the time of mineralization of the organic load (COD)
Organic matter is all matter chemically formed around carbon as its fundamental atoms. When we speak of organic matter we refer to that which is linked to life: that which makes up the bodies of living beings, as well as most of their substances and waste materials.
Figure 25 shows the average COD reduction with respect to ozonation time (hours). In general, as the operating time increases, the concentration of chemical oxygen demand decreased. Note that only 2 hours of contact between the wastewater and ozone are necessary to reach the maximum percentage decrease due to the high solubility and reactivity of the dissolved ozone in this time period and also because the organic matter is easier to degrade. The decomposition of ozone in water occurs by recombination with itself to transform back into oxygen. This decomposition is a function of temperature, the incidence of UV rays and the pH of the medium.

Figure 25. Concentration of chemical oxygen demand as a function of time in the oxidation system: ozonation
The tests were conducted at a defined pH between 6 - 8; as the ozone comes into contact with the wastewater and reacts, the pH is maintained between these basic values guaranteeing the
indirect oxidation by means of hydroxyl radicals. Compared to other reported studies, a study demonstrated that high COD reductions (close to 75 %) can be achieved using low ozone concentrations. In this research a maximum of 3 g O3/h was reported, moreover, reductions up to 98 % of the initial COD content were achieved in hospital wastewater characterized with around 1000- 4000 mg/L organic matter.
Phenol decline time analysis
Phenols or phenolic compounds are organic compounds whose molecular structures contain at least one phenol group, an aromatic ring attached to at least one hydroxyl functional group, these compounds have stable molecules making degradation more difficult. Figure 26 shows the average decrease of phenol with respect to the time (hours) of ozonation where it can be observed that it is necessary 5 to 6 hours of residence in the process to reach the maximum percentage of reduction and thus to be in the maximum permissible limit which is governed by resolution 0631 of 2015. The reduction of phenol in this process had an efficiency of 85 % of the initial content of phenol in the hospital wastewater characterized with around 2-3 mg/L of phenolic compounds.
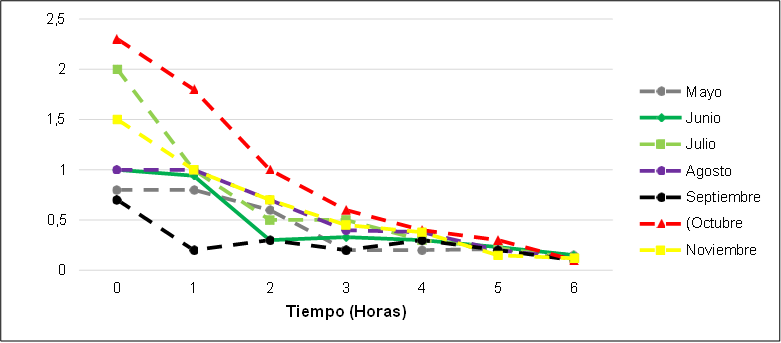
Figure 26. Phenol concentration as a function of time in the oxidation system: ozonation
CONCLUSIONS
According to the reports obtained (2015-2021) it was found that the measurements of most of the physicochemical parameters analyzed are variants, except for phenol measurements. Also, it was observed that, for this time interval most of the parameters did not comply with the stipulations of environmental resolution 0631 of 2015 in Article 14, and as in stability, phenol is an exception by not exceeding the maximum permissible values. Comparing the values of the parameters obtained for the year 2021 with respect to 2020, decreases were observed in the physicochemical parameters of up to 65 % for the rehabilitation and physical medicine unit; while, for Tower A, Tower B and main headquarters only a decrease of 50 % was reached. These favorable values, in the reduction of the pollutant load, are due to the preventive and corrective measures that were developed in each of the assistance processes (ICU, sterilization center, pathology, offices, etc.).
The reduction in the physicochemical parameters was achieved through the implementation of a recirculation system in the contact tanks with the oxidizing agent ozone, as shown in figure 19, this system guarantees the homogenization of the phases present (liquid - gas) in the treatment, with this configuration, yields of over 80 % were achieved. In addition, the residence time necessary for the mineralization of the compounds was evaluated, where it was determined that 2 hours are necessary to reach the oxidation of the organic matter measured as COD and 6 hours to reach values lower than 0,2 mg/L of Phenol.
BIBLIOGRAPHIC REFERENCES
1. Verlicchi P, Galletti A, Petrovic M, Barceló D. Hospital effluents as a source of emerging pollutants: An overview of micropollutants and sustainable treatment options. J Hydrol. 2010;389(3-4):416-28. doi:10.1016/j.jhydrol.2010.06.005
2. Almeida E, Assalin MR, Rosa MA, Durán N. Tratamento de efluentes industriais por processos oxidativos na presença de ozônio. Quím Nova. 2004;27(5):818-24. doi:10.1590/s0100-40422004000500023
3. Balcioğlu IA, Ötker M. Treatment of pharmaceutical wastewater containing antibiotics by O3 and O3/H2O2 processes. Chemosphere. 2003;50(1):85-95. doi:10.1016/S0045-6535(02)00534-9
4. Belzona. Tratamiento de aguas residuales. Belzona Inc.; 2008;1(55):1-15. http://files.bernardo-servin-massieu.com/200000057-b3f9cb4e88/residuales.pdf
5. Belzona. Guía de aplicaciones Belzona en equipos de tratamiento de aguas residuales - Tratamiento de aguas residuales. Belzona Inc.; 2010;40. https://www.belzona.com/es/industries/wastewater.aspx
6. Bes Monge SS, Silva DAM, Bengoa DC. Manual técnico sobre procesos de oxidación avanzada aplicados al tratamiento de aguas residuales industriales. Belzona Inc.; 2016. http://www.cyted.org/sites/default/files/manual_sobre_oxidaciones_avanzadas_0.pdf
7. Camenforte M, Pérez J. Alternativa a la desinfección del agua con cloro: ozonización. 2014;1-20.
8. Centa. Manual de depuración de aguas residuales urbanas. Centa, Secretariado de Alianza por el Agua, Ecología y Desarrollo; 2008;264. http://alianzaporelagua.org/documentos/MONOGRAFICO3.pdf
9. Comisión Nacional del Agua (CONAGUA), Tzatchkov VG, Villagómez IAC. Diseño de lagunas de estabilización. In: Manual de agua potable, alcantarillado y saneamiento. México: CONAGUA; 2015. http://www.conagua.gob.mx/CONAGUA07/Publicaciones/Publicaciones/Libros/10DisenoDeLagunasDeEstabilizacion.pdf
10. González O, Bayarri B, Aceña J, Pérez S, Barceló D. Treatment technologies for wastewater reuse: fate of contaminants of emerging concern. In: The Handbook of Environmental Chemistry. 2015;5-37. https://doi.org/10.1007/698_2015_363
11. Hrenovic J, Ivankovic T, Ivekovic D, Repec S, Stipanicev D, Ganjto M. The fate of carbapenem-resistant bacteria in a wastewater treatment plant. Water Res. 2017;126:232-9. doi:10.1016/j.watres.2017.09.007
12. Benitez FJ, Acero JL, Real FJ, Roldán G. Ozonation of pharmaceutical compounds: rate constants and elimination in various water matrices. Chemosphere. 2009;77(1):53-9. doi:10.1016/j.chemosphere.2009.05.035
13. Lima VC, Prata TS, Landa G, Yannuzzi LA, Rosen RB. Intravitreal triamcinolone and bevacizumab therapy for combined papillophlebitis and central retinal artery occlusion. Retin Cases Brief Rep. 2010;4(2):125-8. doi:10.1097/ICB.0b013e3181ad3957
14. Ministerio de Ambiente y Desarrollo Sostenible. Resolución 631 de 2015. Diario Oficial No. 49.486; 2015. http://www.minambiente.gov.co/images/normativa/app/resoluciones/d1res_631_marz_2015.pdf
15. Manuel J, Navarrete R. Normatividad colombiana en los vertimientos hospitalarios: impactos ambientales y de salud pública. 2016.
16. Ministerio de Ambiente y Desarrollo Sostenible. Resolución 631 de 2015. 2015. http://www.minambiente.gov.co/images/normativa/app/resoluciones/d1res_631_marz_2015.pdf
17. Ministerio del Medio Ambiente. Resolución 01164 de 2002. 2002. http://www.alcaldiabogota.gov.co/sisjur/normas/Norma1.jsp?i=36291
18. Mitcheson L, Maslin J, Meynen T, Morrison T, Hill R, Wanigaratne S. Fundamentals of treatment. In: Applied Cognitive and Behavioural Approaches to the Treatment of Addiction. 2010. https://doi.org/10.1002/9780470661420.ch3
19. Muñoz M, Garcia-Muñoz P, Pliego G, De Pedro ZM, Zazo JA, Casas JA, Rodriguez JJ. Application of intensified Fenton oxidation to the treatment of hospital wastewater: kinetics, ecotoxicity and disinfection. J Environ Chem Eng. 2016;4(4):4107-12. doi:10.1016/j.jece.2016.09.019
20. Muyo C. Procesos biológicos aerobios. Curso sobre tratamiento y reciclaje de aguas residuales industriales mediante soluciones sostenibles; 2016. http://triton-cyted.com/wp-content/uploads/2017/04/Presentaci%C3%B3n.pdf
21. Oikawa S, Tsuda M, Okamura Y, Urabe T. Prefulvene as a stable intermediate at the potential energy surface minimum of the benzene ⇌ benzvalene isomerization process. J Am Chem Soc. 1984;106(22):6751-5. doi:10.1021/ja00334a047
22. Ouarda Y, Tiwari B, Azaïs A, Vaudreuil MA, Ndiaye SD, Drogui P, et al. Synthetic hospital wastewater treatment by coupling submerged membrane bioreactor and electrochemical advanced oxidation process: Kinetic study and toxicity assessment. Chemosphere. 2018;193:160-9. doi:10.1016/j.chemosphere.2017.11.010
23. Penagos DG, López JO, Chaparro TR. Remoción de la materia orgánica y toxicidad en aguas residuales hospitalarias aplicando ozono. DYNA (Colombia). 2012;79(173 Pt I):109-15.
24. Rojas JAR. Calidad del agua. 2009. https://www.belzona.com/es/industries/wastewater.aspx
25. Santiago EB, Calderón Ancona JM. Diseño y construcción de un generador de ozono para aplicaciones de purificación de agua. 2005;120.
26. Shin J, Choi S, Park CM, Wang J, Kim YM. Reduction of antibiotic resistome in influent of a wastewater treatment plant (WWTP) via a chemically enhanced primary treatment (CEPT) process. Chemosphere. 2022;286(P1):131569. doi:10.1016/j.chemosphere.2021.131569
27. Torán J, Blánquez P, Caminal G. Comparison between several reactors with Trametes versicolor immobilized on lignocellulosic support for the continuous treatments of hospital wastewater. Bioresour Technol. 2017;243:966-74. doi:10.1016/j.biortech.2017.07.055
28. Virkutyte J. Treatment of micropollutants in water and wastewater. In: Water Intelligence Online. 2010;9. https://doi.org/10.2166/9781780401447
29. McCabe WL, Smith JC, Harriot P, Colton RH. Operaciones unitarias en ingeniería química. 7.ª ed. México: McGraw-Hill; 2013.
30. Patiño Y, Díaz E, Ordóñez S. Recuperado el 11 de octubre de 2021, de Redalyc: http://www.redalyc.org/articulo.oa?id=323631115001
FINANCING
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORSHIP CONTRIBUTION
Conceptualization: Sara Juliana Jaramillo Arvilla, Julián Diel Urresta Aragón, Natali Lorena Mena Guerrero, Carla Stephanny Cárdenas Bustos.
Data curation: Sara Juliana Jaramillo Arvilla, Julián Diel Urresta Aragón, Natali Lorena Mena Guerrero, Carla Stephanny Cárdenas Bustos.
Formal analysis: Sara Juliana Jaramillo Arvilla, Julián Diel Urresta Aragón, Natali Lorena Mena Guerrero, Carla Stephanny Cárdenas Bustos.
Research: Sara Juliana Jaramillo Arvilla, Julián Diel Urresta Aragón, Natali Lorena Mena Guerrero, Carla Stephanny Cárdenas Bustos.
Methodology: Sara Juliana Jaramillo Arvilla, Julián Diel Urresta Aragón, Natali Lorena Mena Guerrero, Carla Stephanny Cárdenas Bustos.
Project Management: Sara Juliana Jaramillo Arvilla, Julián Diel Urresta Aragón, Natali Lorena Mena Guerrero, Carla Stephanny Cárdenas Bustos.
Resources: Sara Juliana Jaramillo Arvilla, Julián Diel Urresta Aragón, Natali Lorena Mena Guerrero, Carla Stephanny Cárdenas Bustos.
Software: Sara Juliana Jaramillo Arvilla, Julián Diel Urresta Aragón, Natali Lorena Mena Guerrero, Carla Stephanny Cárdenas Bustos.
Supervision: Sara Juliana Jaramillo Arvilla, Julián Diel Urresta Aragón, Natali Lorena Mena Guerrero, Carla Stephanny Cárdenas Bustos.
Validation: Sara Juliana Jaramillo Arvilla, Julián Diel Urresta Aragón, Natali Lorena Mena Guerrero, Carla Stephanny Cárdenas Bustos.
Visualization: Sara Juliana Jaramillo Arvilla, Julián Diel Urresta Aragón, Natali Lorena Mena Guerrero, Carla Stephanny Cárdenas Bustos.
Writing - original draft: Sara Juliana Jaramillo Arvilla, Julián Diel Urresta Aragón, Natali Lorena Mena Guerrero, Carla Stephanny Cárdenas Bustos.
Writing - proofreading and editing: Sara Juliana Jaramillo Arvilla, Julián Diel Urresta Aragón, Natali Lorena Mena Guerrero, Carla Stephanny Cárdenas Bustos.