doi: 10.56294/evk202249
REVIEW
Advanced Treatment of Hospital Wastewater using POAs: Evaluation and Technological Application
Tratamiento Avanzado de Aguas Residuales Hospitalarias mediante POA’s: Evaluación y Aplicación Tecnológica
Sara Juliana Jaramillo Arvilla1, Julián Diel Urresta Aragón1, Natali Lorena Mena Guerrero1, Carla Stephanny Cárdenas Bustos1
1Universidad de Pamplona, Facultad de Ingenierías y Arquitectura, Programa de Ingeniería Química, Norte de Santander. Pamplona, Colombia.
Cite as: Jaramillo Arvilla SJ, Urresta Aragón JD, Mena Guerrero NL, Cárdenas Bustos CS. Advanced Treatment of Hospital Wastewater using POAs: Evaluation and Technological Application. eVitroKhem. 2022; 1:49. https://doi.org/10.56294/evk202249
Submitted: 29-01-2021 Revised: 12-01-2022 Accepted: 18-07-2022 Published: 19-07-2022
Editor: Prof.
Dr. Javier Gonzalez-Argote ![]()
ABSTRACT
Introduction: the study addressed the environmental problems associated with wastewater generated by hospitals, which contains contaminants that are difficult to remove using conventional methods. In Colombia, current regulations established strict limits for its discharge, which prompted the search for more effective technologies such as Advanced Oxidation Processes (AOPs).
Development: the research described the classification and composition of hospital liquid waste, as well as the physicochemical parameters required by Resolution 0631 of 2015. Subsequently, different traditional treatments (primary, secondary, and tertiary) were explored, and AOPs were examined in greater depth, including ozonation, the Fenton process, and photocatalysis. These methods used highly oxidizing agents, such as hydroxyl radicals, capable of degrading recalcitrant compounds present in hospital effluents. The operating conditions and operating principles of each technique were analyzed, as well as their technical and economic feasibility.
Conclusions: the study concluded that POAs represented an effective solution for the tertiary treatment of hospital wastewater. The technologies evaluated significantly improved parameters such as COD, BOD5, and total suspended solids. However, their effectiveness depended on specific operating variables such as pH and oxidant dosage. It was highlighted that the integration of POAs with biological treatments could optimize results and reduce operating costs, promoting more sustainable environmental management in the healthcare sector.
Keywords: Hospital Wastewater; Advanced Oxidation; Ozonation; Fenton Process; Photocatalysis.
RESUMEN
Introducción: el estudio abordó la problemática ambiental asociada a las aguas residuales generadas por hospitales, las cuales contienen contaminantes difíciles de eliminar mediante métodos convencionales. En Colombia, la normativa vigente estableció límites estrictos para su vertimiento, lo que impulsó la búsqueda de tecnologías más eficaces como los Procesos de Oxidación Avanzada (POA’s).
Desarrollo: la investigación describió la clasificación y composición de los residuos líquidos hospitalarios, así como los parámetros fisicoquímicos exigidos por la Resolución 0631 de 2015. Posteriormente, se exploraron los diferentes tratamientos tradicionales (primarios, secundarios y terciarios) y se profundizó en los POA’s, entre ellos la ozonización, el proceso Fenton y la fotocatálisis. Estos métodos emplearon agentes altamente oxidantes, como el radical hidroxilo, capaces de degradar compuestos recalcitrantes presentes en los efluentes hospitalarios. Se analizaron las condiciones operativas y los principios de funcionamiento de cada técnica, así como su viabilidad técnica y económica.
Conclusiones: el trabajo concluyó que los POA’s representaron una solución eficaz para el tratamiento terciario de aguas residuales hospitalarias. Las tecnologías evaluadas lograron mejorar significativamente parámetros como la DQO, la DBO5 y los sólidos suspendidos totales. No obstante, su efectividad dependió de variables operativas específicas como el pH y la dosis de oxidantes. Se destacó que la integración de POA’s con tratamientos biológicos podría optimizar los resultados y reducir costos operativos, promoviendo una gestión ambiental más sostenible en el sector salud.
Palabras clave: Aguas Residuales Hospitalarias; Oxidación Avanzada; Ozonización; Proceso Fenton; Fotocatálisis.
INTRODUCTION
Hospitals and health care centers generate large volumes of wastewater on a daily basis as a result of their multiple care, administrative and laboratory processes. This wastewater contains a complex mixture of organic and inorganic compounds, pathogenic microorganisms, unmetabolized drugs, radioactive materials and potentially toxic chemicals, making it a major source of pollution to the environment if not properly treated. Because of its hazardous nature, this type of wastewater cannot be treated efficiently by the conventional systems used in municipal treatment plants, which poses a significant challenge in terms of both public health and environmental sustainability.
In Colombia, current environmental regulations, in particular Resolution 0631 of 2015 of the Ministry of Environment and Sustainable Development, establishes maximum permissible limits for various physicochemical parameters in wastewater discharges generated by human health care activities. This regulation seeks to prevent contamination of water bodies, protect biodiversity and ensure the health of communities exposed to these discharges. However, compliance with these standards requires the application of advanced technologies capable of effectively removing the pollutants present in these effluents.
In this context, Advanced Oxidation Processes (AOPs) have gained relevance as a viable and efficient alternative for the tertiary treatment of hospital wastewater. These processes are based on the generation of highly reactive species, such as hydroxyl radicals (-OH), which allow the complete or partial degradation of recalcitrant pollutants. Techniques such as ozonation, the Fenton process and photocatalysis have proven to be effective in the mineralization of organic matter, the elimination of pathogens and the reduction of critical parameters such as COD and BOD.
The purpose of this work is to analyze the composition and characteristics of the wastewater generated in a hospital institution and to evaluate the applicability of advanced oxidation technologies in its treatment. The research is framed within the need to implement innovative solutions to improve the quality of the final effluent, ensure compliance with environmental regulations and minimize negative impacts on the environment, thus contributing to the integrated management of water resources in the health sector.
DEVELOPMENT
Theoretical framework
Hospital Wastewater
Hospitals and medical centers in general are an important source of effluents with hazardous and infectious waste for the environment, generally the waste streams are classified into 3 classes, namely: municipal waste which is composed of non-hazardous materials such as kitchen waste and recyclables in which are: paper, cardboard and plastics; medical waste, these are included in hazardous and infectious waste such as anatomopathological waste, surgery waste and materials such as needles, broken glasses liquid waste including pharmaceutical, chemical and biological waste from laboratories; and radioactive waste.
Hospitals require large volumes of water per day, generating a similar volume of wastewater containing pathogenic microorganisms, metabolized or unmetabolized drugs, toxic compounds, among others, which are discharged treated or untreated into the water, affecting its quality and endangering health.(1)
Regulations
The Ministry of Environment and Sustainable Development presents in 2015 Resolution 0631,(2) which aims to reduce and control polluting substances that reach rivers, reservoirs, lagoons, natural or artificial water bodies of fresh water, and the public sewage system, to thus, help the improvement of water quality and work on the environmental recovery of the country's waterways.(3) Table 1 shows the parameters evaluated by the current standard, where the units of each parameter and the maximum permissible limits for each of them are specified.
|
Table 1. Parameters of resolution 0631(2) |
||
|
Parameter |
Units |
Human health care activities - medical care with and without hospitalization |
|
General |
||
|
pH |
pH UNITS |
6,00-9,00 |
|
Chemical Oxygen Demand (COD) |
mg/O2 |
200,00 |
|
Biochemical Oxygen Demand (BOD5) |
mg/O2 |
150,00 |
|
Total Suspended Solids (TSS) |
mg/L |
50,00 |
|
Sedimentable Solids (SSED) |
mg/L |
5,00 |
|
Fats and Oils |
mg/L |
10,00 |
|
Phenols |
mg/L |
0,20 |
|
Formaldehyde |
mg/L |
|
|
Active Substances to Methylene Blue (SAAM) |
mg/L |
Analysis and Reporting |
|
Phosphorus Compounds |
||
|
orthophosphates (PO(3-) 4) |
mg/L |
Analysis and Report |
|
Total Phosphorus (P) |
mg/L |
Analysis and Report |
|
Nitrogen Compounds |
||
|
Nitrates (N-NO3- ) |
mg/L |
Analysis and Report |
|
Nitrites (N-NO2-) |
mg/L |
Analysis and Report |
|
Ammoniacal Nitrogen (N-NH3) |
mg/L |
Analysis and Report |
|
Total Nitrogen (N) |
mg/L |
Analysis and Report |
|
Ions |
||
|
Total Cyanide (CN-) |
mg/L |
0,50 |
|
Metals and Metalloids |
||
|
Cadmium (Cd) |
mg/L |
0,05 |
|
Chromium (Cr) |
mg/L |
0,50 |
|
Mercury (Hg) |
mg/L |
0,01 |
|
Silver (Ag) |
mg/L |
Analysis and Reporting |
|
Lead (Pb) |
mg/L |
0,10 |
|
Other parameters for analysis and reporting |
||
|
Total Acidity |
mg/L CaCO3 |
Analysis and Reporting |
|
Total Alkalinity |
mg/L CaCO3 |
Analysis and Report |
|
Calcium Hardness |
mg/L CaCO3 |
Analysis and Report |
|
Total Hardness |
mg/L CaCO3 |
Analysis and Report |
|
True Color (Absorbance measurements at the following wavelengths: 436 nm 525 nm and 620 nm) |
m-1 |
Analysis and Report |
Parameters evaluated in the standard
The following is a description of each of the parameters evaluated by Resolution 631 of 2015 for human health care activities-medical care with or without hospitalization.(2)
General
· pH. It is a measure of acidity or alkalinity of an aqueous dilution that is defined as the logarithm of the activity or molar concentration of hydrogen ions in water, expressed as the negative algorithm of the molar concentration of hydrogen ion.(4)
· Demanda Chemical Oxygen Concentration (COD). It is the equivalent amount of oxygen required to mineralize the organic matter present in a sample. A strong oxidant such as potassium permanganate is used.(5)
· Demanda Biochemical Oxygen (BOD5): Amount of oxygen required by bacteria to carry out the degradation process of organic matter that serves as food for bacteria and whose oxidation generates energy under aerobic conditions.(5)
· Sólidos Total Suspended Solids (TSS). Particulate matter that remains suspended in water as colloids due to water movement.(4)
· Sedimentable Solids (SSED). Volume of solid particles that are deposited by the force of gravity in a container where the liquid remains immobile for 60 min.(4)
· Fats and oils. Substances of vegetable or animal origin, esters formed by fatty acid molecules and a glycerol molecule, can be solids (fats) or liquids (oils).(4)
· Fenoles. Phenols or phenolic compounds are organic compounds whose molecular structures contain at least one phenol group, an aromatic ring attached to at least one hydroxyl functional group, common compounds in effluents from the petroleum industry, coal, chemical plants, explosives factories, resin factories and others.
· Substances Methylene Blue Active Substances (MAAS). Methylene blue active substances (MBAS) is a cationic dye, it transfers methylene blue from an aqueous solution to an immiscible liquid at equilibrium. This occurs during the formation of an ionic pair between the anion (SAAM) and the methylene blue cation. The intensity of the resulting color is a measure of the substances active to methylene blue.(4)
Phosphorus compounds
· Orthophosphates (PO4). Advanced phosphates defined as inorganic salt of phosphoric acid.
· Total Phosphorus (P). Total phosphorus is the sum of all existing forms of phosphorus: orthophosphate or phosphates, condensed phosphates and organic phosphorus.
Nitrogen compounds
Biomolecules containing nitrogen, either macromolecules or waste products such as nitrates (NO3), nitrites (NO2), and ammoniacal nitrogen (NH3), are called nitrogenous substances or compounds.
Ions
Total Cyanide (CN-). Refers to all groups (CN-) in chemical compounds that can be determined as cyanide ion. Cyanides are potentially toxic compounds and upon a change in the pH of the medium can release hydrocyanic acid, a compound of maximum toxicity to humans.
Metals and metalloids
Cadmium (Cd), Chromium (Cr), Mercury (Hg), Silver (Ag), Lead (Pb). As important constituents of many waters, any cation having an atomic weight greater than 23 g/mol (corresponding to the atomic weight of sodium) is considered a heavy metal; thus, wastewater contains a large number of different heavy metals. These include nickel, manganese, lead, chromium, cadmium, zinc, copper, iron and mercury, among others. All of them are classified as pollutants and, due to their toxic nature, they must be taken into consideration because they have a negative impact on conventional biological treatments as well as on the receiving ecosystems.
Other parameters for analysis and reporting
· Total Acidity (mg/L). refers to the presence of substances that can be dissociated in water and that as a product of dissociation generate the hydronium ion (H3O+), such as strong acids, weak and medium strength acids; also the presence of certain metallic cations such as Fe (III) and Al (III) contribute to the acidity of the medium.(4)
· Total Alkalinity. Water's capacity to neutralize acids or accept protons. This represents the sum of the bases that can be titrated in a sample of water.(4)
· Total Hardness (mg/L). Indicates the total amount of alkaline earth ions (group 2) present in the water and constitutes a quality parameter of water of domestic or industrial interest. It involves all the polyvalent metal ions that may be present in the sample.(4)
· Hardness Calcium (Ca2 + and Mg2 +). quantifies only the hardness due to the effect of the calcium ion.(4)
· Real Color (Absorbance measurements at the following wavelengths. 436 nm, 525 nm and 620 nm). The color in water results from the presence in solution of different substances such as natural metallic ions substances such as natural metal ions, humus and dissolved organic matter. The expression true color is considered to be the color of water from which turbidity has been removed.
Wastewater treatment
The purpose of wastewater decontamination treatments is to mitigate and reduce the level of contamination in order to obtain an effluent that does not generate negative environmental impacts, in compliance with current environmental regulations. The traditional treatments are mechanical or also called physicochemical, which are the set of primary, secondary and tertiary treatments.(6)
Primary treatment
The objective of primary treatment is to remove a significant fraction of suspended solids and floating material from wastewater by sedimentation.(7) The suspended solids removed are organic in nature and therefore contribute to the BOD (biochemical oxygen demand) of the sludge. The floating material can include oil, grease, rags, among others.(8)
On the other hand, chemically enhanced primary treatment refers to a process that uses chemicals for coagulation, flocculation and precipitation of particles.(9)
Coagulation. In this process, destabilization of electrostatically charged colloidal particles occurs. Coagulation is a process that increases the tendency of particles to aggregate one after the other to form larger particles and thus precipitate rapidly, which can be aided by the use of coagulants such as alum (aluminum sulfate) and iron chloride. Some organic polymers and clay materials are also used to stimulate this process. Once coagulated, larger particles are easier to remove than smaller ones. The larger particles become a material called floc. This fluffy material has a larger surface area and helps more in the clarification process by trapping smaller particles on its surface.(10)
Flocculation. A complement to coagulation, floc formation as a result of collision and adhesion between coagulated particles consists of the agitation of the coagulated mass, which serves in the growth and agglomeration of coagulated particles.
which serves in the growth and agglomeration of the newly formed flocs in order to increase the size and weight necessary for easy sedimentation.(10)
Sedimentation. Mechanical separation of solids or liquid droplets through a fluid (at rest or in motion) by the action of gravitational forces, in wastewater treatment is one of the most used unitary processes, because the specific weight of the suspended particles is greater than that of water, sedimentation is essentially a purely physical phenomenon and this operation is used for the removal of sands with the main objective of obtaining a clarified effluent, but it is evident that it is necessary to produce a sludge whose concentration of solids allows easy treatment and handling.(11)
Secondary treatment
Secondary treatment is the final treatment stage prior to disinfection, usually consisting of biological treatment of primary effluent wastewater. The objectives of secondary treatment are to decrease the biochemical oxygen demand (BOD5) and suspended solids in the effluent to acceptable levels.
Secondary wastewater treatment is designed to break down and evacuate material from wastewater before returning it to lakes, streams, rivers, and oceans.(6)
Aerobic treatments. Treatments with aeration are used to decompose organic matter by the action of microorganisms and oxygen present in the air converting to carbon dioxide and other oxidized species.(12)
Anaerobic treatments. Anaerobic biodegradation consists of the transformation, by means of microorganisms, in the absence of oxygen, of organic matter in wastewater into reduced gaseous compounds such as methane, ammonia and sulfuric acid, in addition to carbon dioxide. In this treatment, closed reactors are used in a hermetic process.(13)
Mixed treatments. These are a mixture of aerobic and anaerobic treatments, either in a continuous or alternating
alternating or both at the same time.(13)
Facultative treatments. These biological treatments use so-called facultative organisms and are not affected by the presence of oxygen in the process.(13)
Tertiary treatment (advanced oxidation processes)
When the effluent from secondary treatment does not meet regulations or discharge requirements, additional treatment is necessary to reduce the levels of specific pollutants. This is generally referred to as advanced treatment or tertiary treatment. Advanced treatment processes are used for removal of nutrients such as nitrogen and phosphorus, removal of residual total suspended solids, removal of specific heavy or inorganic metals, and removal of emerging pollutants of concern, among others.(6)
Advanced oxidation processes (AOP's) are those tertiary treatments that are based on the generation of strongly oxidizing species whose main objective is the removal of soluble non-biodegradable compounds present in wastewater, these consist of chemical oxidation under mild conditions of pressure and temperature, until the complete mineralization of pollutants. If the process is sufficiently prolonged or remaining in intermediate oxidation states that allow a coupling with other purification methods such as biological processes.
POA's rely on physicochemical processes capable of originating important changes in the chemical structure of pollutants, involving the generation and use of powerful transient species, mainly the hydroxyl radical (OH-), the generation of radicals is performed from oxygen, hydrogen peroxide and supported catalysts, so the reaction by-products are only water and carbon dioxide.(14)
POA's can be classified in different ways, as shown in table 2, as non-photochemical processes and photochemical processes depending on the participation of light in the process.
|
Table 2. Most commonly used AOPs(14) |
|
|
Non-photochemical processes |
Photochemical processes |
|
Ozonation |
Vacuum ultraviolet |
|
Ozonation with O3/H2O2 |
UV/H2O2 |
|
|
UV/O3 |
|
Fenton (Fe+2/H2O2) and related processes |
|
|
electrochemical oxidation |
UV/O3/H2O2 |
|
Radiolysis and electron beam treatment |
Homogeneous Solar Photocatalysis: Photo-Fenton and related Heterogeneous: Photocatalysis with TiO2 |
|
Non-thermal plasma |
|
|
Oxidation in subcritical and supercritical water |
|
|
Gamma irradiations |
|
|
Electron accelerators |
|
POA's use expensive reagents such as hydrogen peroxide or ozone, or consume large amounts of energy (UV, among others),(14) has confirmed that when combined with other processes such as adsorption or biological processes they acquire their potential in terms of economic efficiency due to savings in chemicals and/or energy.
Ozonation
The ozonation process is a non-photochemical process where highly reactive species are generated mainly the hydroxyl radical in sufficient quantities to interact with organic compounds in the medium. Ozone can be used for mineralization (TOC removal) of organic molecules, although in many cases it involves the use of high doses of ozone and the overpricing of the process.
In ozonation, the effectiveness depends on the chemical and biological contamination of the water to be treated, the contact time and the ozone concentration.
In figure 1, we can observe the components of the ozonation process, which includes the preparation of the feed gas, ozone generation, ozone contact, which is usually performed either by bubble diffusers or by Venturi type injectors, and ozone destruction, which is performed by thermal destruction or by catalytic destruction with palladium or palladium oxide catalysts, and ozone destruction, which is performed by thermal destruction or by catalytic destruction with palladium or palladium oxide catalysts, palladium, nickel oxide or manganese catalysts.
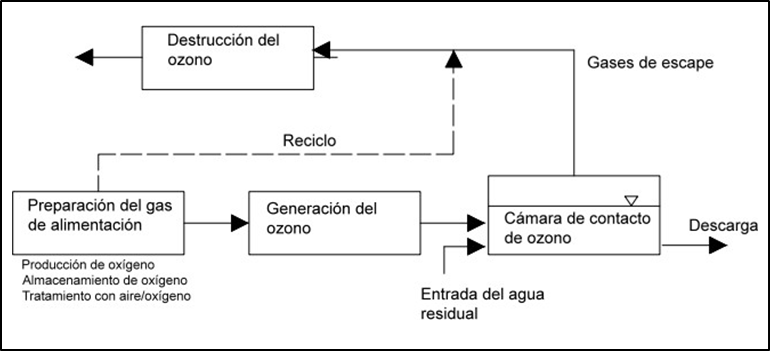
Figure 1. Ozonation process
Ozone is produced in an ozone generator. The feed gas can be air or pure oxygen. Approximately 1 to 10 percent of the oxygen flowing through the electrodes is transformed into ozone.(15)
There are different methods for ozone production that can be divided into three categories, depending on whether they are used: corona discharge, electrochemical discharge and UV methods. Among them, corona discharge or also known as "silent electrical discharge", is the most widely used generation method.
Ozone in its industrial use, either from air or pure oxygen, is obtained by high-voltage alternating electrical discharge. On the other hand, to avoid the formation of an electric arc (silent electric discharge), the two electrodes are separated by a dielectric medium, usually glass. The corona discharge between the two electrodes causes an electron to flow through the discharge gap, as shown in figure 2.
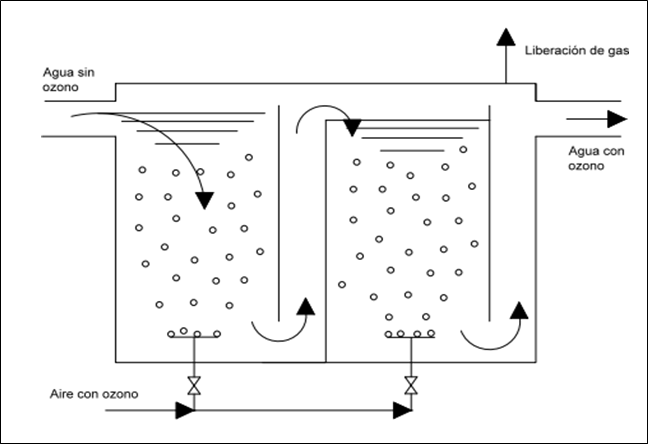
Figure 2. Ozone production(15)
These corona discharge devices generate a large amount of heat (about 80 to 95 percent of the energy is converted to heat) that could lead to the decomposition of the ozone produced. Thus, the cooling system of ozonizers is very important to maintain a constant temperature of the gas inside the discharge chamber. This system is usually performed by circulating a refrigerant that could be: water or air.(15) It is necessary for the ozone to come into contact with the water to be treated and to be dispersed as thinly as possible. Normally, this is done through fine bubble diffusers in contact chambers. The most commonly used types of diffused bubble contact chambers are: positive pressure injection, negative pressure injection, mechanical agitation and fixed bed towers. Baffled chamber diffusers appear to be the most common.
A contact chamber with baffles is shown in figure 3. A typical chamber usually has several compartments in series with bubble diffusers at the bottom. In the first compartment, the water flows downward in the opposite direction of the bubbles, which rise, and in the second compartment the water flows upward. The chambers are covered to prevent ozone leakage and increase the ozone partial pressure in the chamber. The additional chambers ensure the contact time between ozone and water.(16)
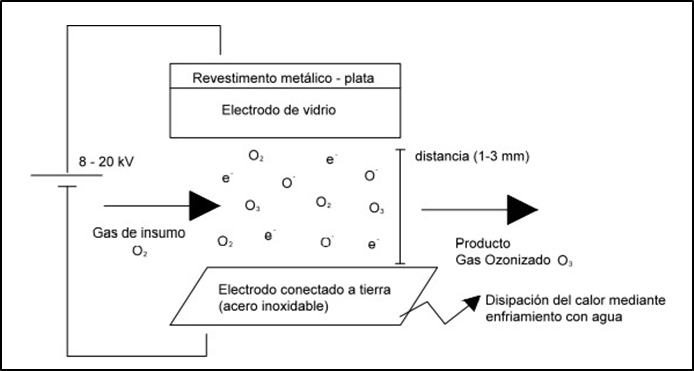
Figure 3. Contact chamber with baffles(16)
Generally, there is a main reactor where the Fenton process occurs in batch mode, usually in a non-pressurized tank, under atmospheric conditions, which has a series of external pumps to be able to add the pH adjustment agent (either acid or base), and to dose the iron (II) sulfate and hydrogen peroxide (35-50 %) in solution. After filling the oxidation tank with the wastewater, the addition of the reagents is carried out as can be seen in figure 4, through this procedure.
· Add acid or base to adjust the pH to 2,5-4 (2,8 ideally).
· Add the reagents little by little so that no sudden changes in pH or temperature occur temperature.
· Add the iron (II) sulfate and then H2O2.
· It is passed to a neutralization tank to add a base.
· Precipitate the iron hydroxide in the form of slurry.
· Coagulate the sludge in a flocculation tank.
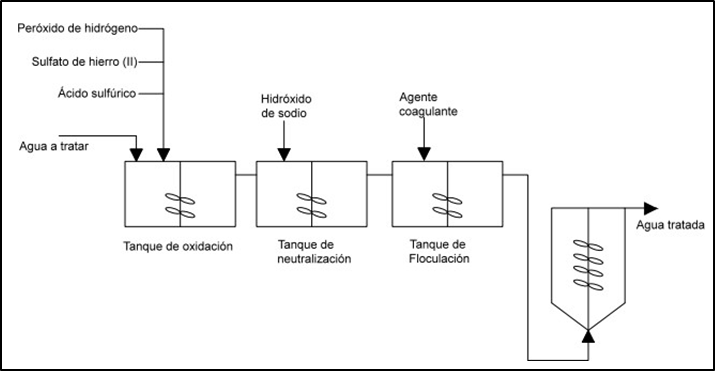
Figure 4. Industrial scheme of the Fenton process(14)
Photocatalysis
Photocatalysis employs UV and/or visible radiation as the driving force for water treatment. This process causes the acceleration of a photochemical reaction through the presence of a catalyst (sensitizer), which results in the removal of organic matter and heavy metals dissolved in the wastewater. Within photocatalysis there are two types of techniques: heterogeneous processes, mediated by a semiconductor as a catalyst, and homogeneous processes where the system is used in a single phase (i.e., dissolved catalyst). Photocatalysis is applied when the contaminant itself is not capable of capturing photons, and therefore requires the use of a sensitizer (the catalyst) that absorbs the radiant energy and accelerates oxidation. The application of solar radiation for photochemical processes is only possible with certain catalysts and depending on the configuration of the photo-reactor, such as dissolved iron cations or titanium dioxide in solid phase, for heterogeneous or homogeneous catalysis, respectively. Through this technology, oxidants such as hydroxyl radical are generated which, in aqueous media, react with organic pollutants by degrading them to carbon dioxide, water and other salts. In addition, water disinfection can be promoted.(14,17)
CONCLUSIONS
The proper management of hospital wastewater represents one of the main environmental and sanitary challenges in the current context, due to the presence of recalcitrant compounds, pathogenic microorganisms, pharmaceutical residues and heavy metals that are not efficiently removed by conventional treatments. The complex nature of these effluents requires the implementation of advanced technological solutions that guarantee the effective purification of the water before its final disposal in receiving bodies or its reincorporation into municipal treatment systems.
Colombian regulations, through Resolution 0631 of 2015, establish clear parameters for the discharge of wastewater generated in the health sector, which forces hospital institutions to adopt more rigorous and sustainable strategies in their environmental management. In this sense, Advanced Oxidation Processes (AOP's) emerge as a fundamental tool in the tertiary treatment of these effluents, by allowing the degradation of persistent pollutants that escape traditional physicochemical and biological processes.
Among the technologies evaluated, ozonation, the Fenton process and photocatalysis demonstrated high potential for the removal of specific pollutants such as non-biodegradable organic matter, color, phenols and certain heavy metals. These techniques, although they require controlled conditions and may involve high operating costs, offer important advantages by achieving greater mineralization of organic compounds and significant improvement in key parameters such as COD, BOD5, and total suspended solids.
However, the efficiency of these processes is subject to factors such as pH, oxidant concentration, contact time and reactor configuration, which requires an adequate characterization of the effluent and a technical design adapted to the specific needs of each institution. The possibility of integrating AOPs with biological treatment or adsorption systems to optimize efficiency and reduce costs is also highlighted, promoting more sustainable and economically viable hybrid schemes.
In conclusion, the use of advanced technologies such as AOPs represents an effective and environmentally responsible technical solution for the treatment of hospital wastewater. Their proper implementation would contribute significantly to the protection of water resources, regulatory compliance and the improvement of environmental quality in the Colombian health sector.
REFERENCES
1. Ministerio del Medio Ambiente. Resolución 01164 de 2002 [Internet]. 2002 [citado 2021 jun 15]. Disponible en: http://www.alcaldiabogota.gov.co/sisjur/normas/Norma1.jsp?i=36291
2. Ministerio de Ambiente y Desarrollo Sostenible. Resolución 631 de 2015 [Internet]. 2015 [citado 2021 jun 15]. Disponible en: http://www.minambiente.gov.co/images/normativa/app/resoluciones/d1res_631_marz_2015.pdf
3. Manuel J, Navarrete R. Normatividad colombiana en los vertimientos hospitalarios: impactos ambientales y de salud pública. 2016.
4. Rojas JAR. Calidad del agua [Internet]. 2009 [citado 2021 ago 25]. Disponible en: https://www.belzona.com/es/industries/wastewater.aspx
5. Oikawa S, Tsuda M, Okamura Y, Urabe T. Prefulvene as a stable intermediate at the potential energy surface minimum of the benzene benzvalene isomerization process. J Am Chem Soc. 1984;106(22):6751–5. doi:10.1021/ja00334a047
6. Mitcheson L, Maslin J, Meynen T, Morrison T, Hill R, Wanigaratne S. Fundamentals of treatment. In: Applied Cognitive and Behavioural Approaches to the Treatment of Addiction [Internet]. 2010 [citado 2021 may 2]. Disponible en: https://doi.org/10.1002/9780470661420.ch3
7. Centa. Manual de depuración de aguas residuales urbanas [Internet]. Centa, Secretariado de Alianza por el Agua, Ecología y Desarrollo; 2008 [citado 2021 sep 25];264. Disponible en: http://alianzaporelagua.org/documentos/MONOGRAFICO3.pdf
8. Bes Monge SS, Silva DAM, Bengoa DC. Manual técnico sobre procesos de oxidación avanzada aplicados al tratamiento de aguas residuales industriales [Internet]. Belzona Inc.; 2016 [citado 2021 sep 7]. Disponible en: http://www.cyted.org/sites/default/files/manual_sobre_oxidaciones_avanzadas_0.pdf
9. Shin J, Choi S, Park CM, Wang J, Kim YM. Reduction of antibiotic resistome in influent of a wastewater treatment plant (WWTP) via a chemically enhanced primary treatment (CEPT) process. Chemosphere. 2022;286(P1):131569. doi:10.1016/j.chemosphere.2021.131569
10. Belzona. Guía de aplicaciones Belzona en equipos de tratamiento de aguas residuales - Tratamiento de aguas residuales [Internet]. Belzona Inc.; 2010 [citado 2021 sep 7];40. Disponible en: https://www.belzona.com/es/industries/wastewater.aspx
11. McCabe WL, Smith JC, Harriot P, Colton RH. Operaciones unitarias en ingeniería química. 7.ª ed. México: McGraw-Hill; 2013.
12. Muyo C. Procesos biológicos aerobios [Internet]. Curso sobre tratamiento y reciclaje de aguas residuales industriales mediante soluciones sostenibles; 2016 [citado 2021 jun 19]. Disponible en: http://triton-cyted.com/wp-content/uploads/2017/04/Presentaci%C3%B3n.pdf
13. González O, Bayarri B, Aceña J, Pérez S, Barceló D. Treatment technologies for wastewater reuse: fate of contaminants of emerging concern. In: The Handbook of Environmental Chemistry [Internet]. 2015 [citado 2021 may 2];5–37. Disponible en: https://doi.org/10.1007/698_2015_363
14. Muñoz M, Garcia-Muñoz P, Pliego G, De Pedro ZM, Zazo JA, Casas JA, Rodriguez JJ. Application of intensified Fenton oxidation to the treatment of hospital wastewater: kinetics, ecotoxicity and disinfection. J Environ Chem Eng. 2016;4(4):4107–12. doi:10.1016/j.jece.2016.09.019
15. Santiago EB, Calderón Ancona JM. Diseño y construcción de un generador de ozono para aplicaciones de purificación de agua. 2005;120.
16. Camenforte M, Pérez J. Alternativa a la desinfección del agua con cloro: ozonización. 2014;1–20.
17. Torán J, Blánquez P, Caminal G. Comparison between several reactors with Trametes versicolor immobilized on lignocellulosic support for the continuous treatments of hospital wastewater. Bioresour Technol. 2017;243:966–74. doi:10.1016/j.biortech.2017.07.055
FUNDING
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORSHIP CONTRIBUTION
Conceptualization: Sara Juliana Jaramillo Arvilla, Julián Diel Urresta Aragón, Natali Lorena Mena Guerrero, Carla Stephanny Cárdenas Bustos.
Data curation: Sara Juliana Jaramillo Arvilla, Julián Diel Urresta Aragón, Natali Lorena Mena Guerrero, Carla Stephanny Cárdenas Bustos.
Formal analysis: Sara Juliana Jaramillo Arvilla, Julián Diel Urresta Aragón, Natali Lorena Mena Guerrero, Carla Stephanny Cárdenas Bustos.
Research: Sara Juliana Jaramillo Arvilla, Julián Diel Urresta Aragón, Natali Lorena Mena Guerrero, Carla Stephanny Cárdenas Bustos.
Methodology: Sara Juliana Jaramillo Arvilla, Julián Diel Urresta Aragón, Natali Lorena Mena Guerrero, Carla Stephanny Cárdenas Bustos.
Project Management: Sara Juliana Jaramillo Arvilla, Julián Diel Urresta Aragón, Natali Lorena Mena Guerrero, Carla Stephanny Cárdenas Bustos.
Resources: Sara Juliana Jaramillo Arvilla, Julián Diel Urresta Aragón, Natali Lorena Mena Guerrero, Carla Stephanny Cárdenas Bustos.
Software: Sara Juliana Jaramillo Arvilla, Julián Diel Urresta Aragón, Natali Lorena Mena Guerrero, Carla Stephanny Cárdenas Bustos.
Supervision: Sara Juliana Jaramillo Arvilla, Julián Diel Urresta Aragón, Natali Lorena Mena Guerrero, Carla Stephanny Cárdenas Bustos.
Validation: Sara Juliana Jaramillo Arvilla, Julián Diel Urresta Aragón, Natali Lorena Mena Guerrero, Carla Stephanny Cárdenas Bustos.
Visualization: Sara Juliana Jaramillo Arvilla, Julián Diel Urresta Aragón, Natali Lorena Mena Guerrero, Carla Stephanny Cárdenas Bustos.
Writing - original draft: Sara Juliana Jaramillo Arvilla, Julián Diel Urresta Aragón, Natali Lorena Mena Guerrero, Carla Stephanny Cárdenas Bustos.
Writing - proofreading and editing: Sara Juliana Jaramillo Arvilla, Julián Diel Urresta Aragón, Natali Lorena Mena Guerrero, Carla Stephanny Cárdenas Bustos.