doi: 10.56294/evk202226
REVIEW
Lon adsorption behavior (in aqueous solutions) of lanthanum, cerium, and europium in organic adsorbents: review of kinetic, isothermal, and thermodynamic studies
Comportamiento de la adsorción de iones (en soluciones acuosas) de lantano, cerio, y europio en adsorbentes orgánicos: revisión de estudios cinéticos, isotérmicos y termodinámicos
Roxana Alejandra Ramirez Moriano1, Jacqueline Corredor Acuña1
1Universidad de Pamplona, Facultad de Ingenierías y Arquitectura, Departamento de Ingeniería Ambiental, Civil y Química. Pamplona, Colombia.
Cite as: Ramirez Moriano RA, Corredor Acuña J. Lon adsorption behavior (in aqueous solutions) of lanthanum, cerium, and europium in organic adsorbents: review of kinetic, isothermal, and thermodynamic studies. eVitroKhem. 2022; 1:26. https://doi.org/10.56294/evk202226
Submitted: 29-08-2021 Revised: 19-12-2021 Accepted: 03-03-2022 Published: 04-03-2022
Editor: Prof.
Dr. Javier Gonzalez-Argote ![]()
ABSTRACT
Rare earth elements are present in a large number of raw materials for different applications in high technology, including lasers, magnets, fiber optics, X-ray machines, and lamps. Therefore, efforts have been made to find new alternatives that improve the recovery and recycling of these important elements, given that rare earth reserves worldwide are scarce and highly polluting. The aim is to counteract demand and help the environment by using alternative methods and making use of waste at the end of its useful life. Some of this waste is found in water sources from treatment plants and mining, which is significantly dangerous for nature and humans. As a viable alternative, the adsorption method has been chosen as it is an effective and low-cost process. This document aims to analyze different literature on the adsorption of lanthanum, cerium, and europium ions (in aqueous solutions) on organic adsorbents such as biomass and activated carbons, in order to verify whether the adsorption technique proves to be effective for the recovery of these elements, analyzing the kinetic, isothermal, and thermodynamic models. The results obtained confirm that the parameters that depend on adsorption are contact time, pH, and temperature, with a high capacity for removing metal ions. The isotherms most used by the different authors were Langmuir and Freundlich. For the kinetic study, a correlation was found with the activation energy taking place in chemisorption. Most of the literature studied showed that these were spontaneous and endothermic processes.
Keywords: Bioadsorbents; Chemical Equilibrium; Physisorption; Chemisorption; Rare Earths.
RESUMEN
Los elementos de tierras raras están presentes en una gran cantidad de materia prima para diferentes aplicaciones en la alta tecnología, entre ellas se encuentra los láseres, imanes, fibra óptica, máquinas de rayos X y lámparas, por lo que se ha tratado de encontrar nuevas alternativas que mejoren la recuperación y el reciclaje de estos elementos tan importantes, debido a que las reservas de tierras raras en todo el mundo son escasas y son sumamente contaminantes, se busca contrarrestar la demanda y ayudar al medio ambiente acudiendo a métodos alternativos y dándole uso a los desechos al final de su vida útil, algunos de estos desechos se encuentran en fuentes hídricas provenientes de plantas de tratamiento y de minería, lo que es significativamente peligroso para la naturaleza y los seres humanos. Como alternativa viable se ha optado por el método de adsorción ya que es un proceso eficaz y de bajo costo, este documento tiene como objetivo analizar diferente literatura sobre adsorción de iones (en soluciones acuosas) de lantano, cerio, y europio en adsorbentes orgánicos como biomasa y carbones activados, con el fin de verificar si la técnica de adsorción demuestra ser eficaz para la recuperación de estos elementos, analizando los modelos cinéticos, isotérmicos y termodinámicos. Los resultados obtenidos comprueban que los parámetros que dependen de la adsorción son el tiempo de contacto, el pH, y la temperatura teniendo una gran capacidad de remoción de iones metálicos, las isotermas más utilizadas por los diferentes autores fue Langmuir y Freundlich, para el estudio cinético se encontró la correlación con la energía de activación tomando lugar en la quimisorción, la mayoría de la literatura estudiada resultó ser procesos espontáneos y endotérmicos.
Palabras clave: Bioadsorbentes; Equilibrio Químico; Fisisorción; Quimi Sorción; Tierras Raras.
INTRODUCTION
Rare earth elements (REE) are a group of special metals composed of seventeen elements, fifteen of them located in the lanthanide group that contain unique and diverse chemical, magnetic and luminescent properties that make them significantly important in several high-tech industries due to their diverse uses in high-strength permanent magnets, lasers, automotive catalytic converters, fiber optics/superconductors and electronic devices.(1,2) This group of elements is divided into two classes, LREE and HREE, where the former is composed of cerium (Ce), lanthanum (La), neodymium (Nd), praseodymium (Pr), samarium (Sm), europium (Eu), promethium (Pm) and the second includes gadolinium (Gd), terbium (Tb), dysprosium (Dy), thulium (Tm), ytterbium (Yb), lutetium (Lu), holmium (Ho) and erbium (Er).(3)
China currently produces over 90 % of rare earth elements including the Bayan Obo deposit which has a REE reserve of over 57,4 million metric tons but is not fully large enough to mitigate demand around the world, so the rest of the countries face a supply risk, relying on recycling from scrap, industrial waste and end-of-life products, so it will be an absolute necessity to look for alternatives that are cost-effective from the economic and environmental points of view, which caused the implementation of separation and recovery methods to achieve elements with significant purities.(4,5)
There are several methods of REE separation by conventional techniques that carry a significant disadvantage due to their high energy consumption and operating costs, such as purification, co-precipitation, solvent extraction, and ion exchange. Therefore, an alternative method is being studied, including adsorption, for the removal and recovery of metal ions.
Among the applications of heavy rare earth elements are: Optical spectrophotometer wavelength calibration and magnet (Ho), portable X-ray machine,
metal halide lamp and laser (Tm), Nd-based magnet and additives in hard disk drive (Dy), infrared laser, vanadium steel and optical fiber (Er), for light rare earth elements are: Optics, batteries, catalysis (La), Chemical applications, dyes, catalysis (Ce), lasers, color television, lighting, medical applications (Eu), this makes them primary elements for recovery seeking to take advantage of existing waste.(6,7)
The two main drivers of demand for REE are the overall economic growth rate and new development in material applications. For example, sustainable technologies that include REE have grown dramatically over the past two decades. The fastest growth has been in demand for new applications, including magnets, phosphors, catalysts, and batteries, which now account for more than 60 percent of demand and are expected to continue growing, driven by substantial investments in clean energy.(8)
Non-recycled REE can cause contamination in waters due to mining outflows or treatment plants, affecting the environment and generating a need for effective elimination of these elements. To remedy the high concentrations found in rivers and streams, a novel treatment such as adsorption is chosen.
This monograph will investigate the adsorption method using organic adsorbents, such as biomass and activated carbons, to remove lanthanum, cerium, and europium ions from aqueous solutions, highlighting these new ecological alternatives as they help mitigate adverse environmental impacts. It will focus on adsorption isotherms, kinetic models, and thermodynamic parameters (ΔGº, ΔHº, ΔSº), as well as adsorption capacity (qe) and equilibrium times, reviewing the literature on the subject over the last five years.
How do organic adsorbents such as biomass and activated carbons contribute to the efficient removal of lanthanum, cerium and europium ions in aqueous solutions, considering kinetic, isothermal models and thermodynamic parameters according to recent studies?
Objective
To conduct a study on the adsorption of lanthanum, cerium, and europium ions (in aqueous solutions) on organic adsorbents such as biomass and activated carbons, focusing on kinetic, isothermal models and thermodynamic parameters, reviewing the last five years of literature on the subject.
DEVELOPMENT
Rare Earth Elements (REE)
Rare earth elements comprise fifteen lanthanides, yttrium, and scandium, which are found in more than 250 minerals worldwide. REEs are utilized in a range of high-tech applications across various industries, including electrical and electronics, automotive, renewable energy, medical, and defense. Therefore, the demand for REE in the global market is increasing daily due to the growing demand from various sectors, including emerging economies and green technology.(9)
Lanthanum, Cerium, and Europium belong to the lanthanide group. This group is composed of fifteen elements, which are divided into two (light and heavy). The light ones are more abundant in nature, so the heavy ones are said to be in a critical state, as shown in figure 1. The distribution can be observed in the periodic table.

Figure 1. Distribution of the lanthanide group in the periodic table of chemical elements(10)
REE are found together in nature because of their similar physicochemical properties; they are all trivalent ions (oxidation state +3), except Ce+4 and Eu+2. This similarity allows the substitution of REE elements among themselves in various crystal lattices, which is why there are multiple elements in this group.
This is why multiple elements of this group occur in the same mineral. This group of chemical elements has specific magnetic, optical, and conductive properties that make them unique and, therefore, required by the industry.(10)
Rare earth elements are not as rare as their name suggests; most LREE have similar abundances in the Earth’s crust as much as most industrial metals, such as chromium, nickel, copper, zinc, tin, tungsten, or lead.(9,11)
These elements have different applications for example europium has been used as a dopant in laser crystals and as a fluorescent material in cathode ray tube televisions and fluorescent lamps, lanthanum is often used in batteries, catalysis among others, cerium is used in dyes, and in catalysis.(7,12) Some of the most important characteristics for these elements are described in table 1.
|
Table 1. Characteristics of the studied rare earth elements La, Ce, and Eu |
|||
|
Characteristics |
Cerium |
Lanthanum |
Europium |
|
Atomic symbol |
Ce |
The |
Eu |
|
Atomic number |
58 |
57 |
63 |
|
Atomic weight (g / mol) |
140,115 |
138,9054 |
151,965 |
|
Melting point (°C) |
795 |
920,9 |
822 |
|
Boiling point (°C) |
3443 |
3456,9 |
1529 |
|
Valences |
+3, +4 |
+3 |
+3, +2 |
|
Properties |
A reactive, gray metal that tarnishes in air and burns when heated. |
Element soft, ductile, silvery-white metallic element that tarnishes rapidly in air. |
It is a ductile metal and has the second lowest melting point and lowest density of all the rare earth elements. |
|
Discoverer |
Wilhelm von Hisinger, J. Jacob Berzelius, Martin Klaproth (1803). |
Carl Gustaf Mosander (1839). |
Paul Émile Lecoq de Boisbaudran (1890). |
|
Reference |
(13) |
(14) |
(12) |
Adsorption fundamentals
Adsorption has received remarkable attention and acceptance due to its low cost, being a simple and environmentally friendly process. IUPAC defines adsorption as “the increase in concentration of a substance at the interface of a condensed layer and a liquid or gaseous one due to the operation of surface forces “. The solute molecules (adsorbate) are characterized as a substance in solid, liquid or gas phase, which will be adsorbed, and the substance on which the adsorbate is adsorbed is named as adsorbent.(15) The adsorption mechanism is described graphically in figure 2.
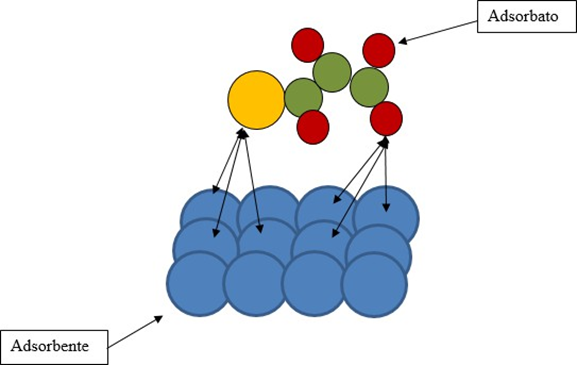
Figure 2. Graphical model of the adsorption process
The adsorption process is classified in two ways, physisorption and chemisorption, these phenomena are related depending on their nature, the first depends on weak forces such as van der Waals forces, usually physical adsorption occurs in multilayers and occupies all adsorption sites; the second depends on the formation of chemical bonds between adsorbate and adsorbent forming a single layer and occupying only one site on the surface, in table 2. A brief summary and differences between these two phenomena are found.(15)
|
Table 2. Differences between physisorption and chemisorption |
||
|
Physisorption |
Chemisorption |
Reference |
|
Reversible |
Irreversible |
(16) |
|
Van der Waals interactions between an adsorbed atom or molecule and molecules on a solid surface. |
Covalent chemical bonds between an adsorbed atom or molecule. |
|
|
Multilayer |
Monolayer |
(13) |
|
Depends linearly on temperature. |
Depends exponentially on temperature (Arrhenius equation). |
(17) |
|
Adsorption enthalpy lower than 40 KJ/mol. |
Adsorption enthalpy higher than 80 KJ/mol. |
(18) |
|
The amount of adsorption on a surface depends on the depends on more on the adsorbed substance than on the adsorbent. |
The amount of adsorption depends on the substance being adsorbed and the adsorbent. |
(18) |
|
Adsorption is only appreciable at temperatures lower than the boiling temperature of the substance being adsorbed. |
Adsorption also occurs at elevated temperatures. |
(18) |
Another way to describe adsorption is as a surface phenomenon. It is defined as the transfer of a mass from a liquid to a solid, the amount of substances adsorbed by a liquid or a solid depends on: the nature of the substance to be adsorbed, the chemical nature of the adsorbent substance, the surface area of the adsorbent substance, (the higher the porosity, the higher the adsorption), the temperature, the concentration of the substance.(18)
Adsorption is a slower mechanism that depends mainly on the composition of the adsorbent. It is generally irreversible due to the large forces that occur between the adsorbate and the adsorbent during the adsorption process. Materials that can be used in the process range from natural substrates or biosorbents, including roots, bark, and leaves, to synthetic sorbents such as activated carbon and metal oxides.(19)
Adsorption studies generally contain adsorption kinetics, isotherms, and thermodynamics. The thermodynamic parameters are described by the change in standard Gibbs free energy (∆G°), change in standard enthalpy (∆H°), and change in standard entropy (∆S°) offering useful information to express adsorption behavior.(20)
The literature that was studied pertains to batch adsorption processes. Geankoplis states that this batch method is used to adsorb solutes from liquid solutions when the quantities treated are small. This batch process can also be used in the pharmaceutical and other industries. As in many other processes, an equilibrium relationship, such as the Freundlich or Langmuir isotherm, and a mass balance are required.(21)
Modeling of adsorption equilibrium
In the process of removing heavy metals, such as rare earth elements, by adsorption, it is crucial to understand and identify a suitable design model for this purpose. Kinetics, isotherms, and thermodynamic parameters such as ΔGº, ΔHº, ΔSº, qe (adsorption capacity), and equilibrium times are taken into account.
As soon as the adsorption process starts, the adsorbate molecules begin to adsorb on the surface (adsorbent) generating a velocity, as the time in the process increases, a decrease in the adsorption velocity and an increase in the desorption velocity begins to be evidenced, this occurs until an equilibrium between the two situations is achieved. The correlation of equilibrium data, either through theoretical or empirical equations, is essential for the practical design and operation of adsorption systems.
The equilibrium formed in this process is also called an adsorption isotherm, providing information such as the amount of adsorbed ions. This is the primary source of information about the adsorption process and is fundamental to optimizing adsorbent use because it describes how an adsorbate interacts with an adsorbent.(22)
The adsorption capacity can be calculated using equation 1. Depending on a mass balance between concentrations, volume, and the amount of adsorbent.
Equation 1. Adsorption capacity
𝑞𝑒 = ( 0- )𝐶𝐶𝑒∗𝑣𝑚
Where: qe is the adsorption capacity (mg /g), C0 and Ce (mg / L) are the initial adsorbate concentration and the equilibrium concentration respectively, v refers to the volume (L) and m is the amount of adsorbate (mg / L) and m is the amount of adsorbate (mg / L).
(L) Moreover, m is the amount of adsorbate (g).(23)
Kinetics and activation energy of adsorption
Largitte L et al.(24) explain the steps that occur in the adsorption process, these are very important because the kinetic models can be adapted depending on the step of the process, these are divided into three, in the first one occurs the external mass transfer of the adsorbate from the solution to the external surface of the adsorbent, in the second one occurs the internal diffusion of the adsorbate to the sorption sites and finally the third stage occurs the adsorption itself. The kinetic model that applies for diffusion in external mass transfer as the slowest process is given by Fick’s law, in contrast to this, internal diffusion as the slowest step can use the Manivela model, finally if the slowest step occurs in adsorption the models that can be applied are pseudo first order, pseudo second order, Elovich and Langmuir that usually applies for the removal of metal ions; Adsorption is usually the slowest process when adsorbate adsorption on the adsorbent is chemical in nature in other words chemisorption.
The pseudo first order and pseudo second order equation are expressed as follows:

Where: qt is the amount adsorbed over time (mg/g), k1 is the pseudo-first-order rate constant (min-1), k2 is the pseudo-second-order rate constant, and t is the time (min).(3)
The adsorption kinetic study is crucial for establishing process design parameters and determining the application of REE recovery, as well as the adsorption rates and activation energy of the process.(25) The activation energy (Ea) indicates the ratio in the adsorption process between the adsorbent and the metal ion, determining whether the reaction occurs physically or chemically. To determine the activation energy, studies are conducted on the variation of adsorption capacity with time at different temperatures.(26)
Chemisorption depends exponentially on temperature, which is reflected in the Arrhenius diagram based on the ratio of the rate constant. The empirical equation is described as:

Where:
k is the kinetic constant, A is the pre-exponential factor, Ea is the activation energy (J /mol), R universal constant of gases (8,314 J/mol.k) and T the temperature (k).(26)
Linearly the Arrhenius equation takes the form:

By Equation 5. The value of the activation energy is found by plotting lnk vs 1/T and the slope of this line takes the value of -Ea/R.(26)
Adsorption isotherms
The study of these isotherms plays an important role because they provide information on the maximum adsorption capacity, mechanism and properties of the adsorbents, these are described by the relationship between the equilibrium concentrations of adsorbate in liquid phase and the amount of adsorbate in equilibrium in solid phase at a given temperature, with these data that provide us could delimit and evaluate the performance of the adsorbents.(17)
There are many isotherm models; among the most prominent are the physical and chemical adsorption models. Chemical isotherms describe the monolayer adsorption process, while physical isotherms represent multilayer adsorption. Among the best-known are the Langmuir model, the Freundlich, Temkin, Sips, and Volmer models, among others. In this study, only the Langmuir, Freundlich and Temkin models were studied because they are the most commonly reported models in the literature.(17)
Langmuir isotherm
The Langmuir isotherm is the isotherm widely reported in the literature for the adsorption of metal ions, dyes, drugs, and other forms of organic contaminants on bioadsorbents and abiotic adsorbents, followed by the Freundlich model. This model has the characteristics listed in table 3.
The following equation represents the Langmuir isotherms:

Where:
qe refers to the amount of adsorption (mg adsorbate/g adsorbent), qm is the maximum adsorption capacity (milligrams/grams), bL is the Langmuir constant (Liters/milligrams) and Ce is the value of the concentration at equilibrium (milligram/liter). This equation is solved by the nonlinear regression method.

Figure 3. Adsorption mechanism Langmuir model(27)
This model belongs to chemical adsorption because it occurs in the monolayer, this means that the adsorbate molecules are adsorbed on the adsorption sites of the adsorbents.(27) The schematic representation of this model can be seen in figure 3.
Freundlich isotherm
It was the first isotherm model proposed by Herber Freundlich in 1906, he determined it by experimental results. This model describes the adsorption equilibrium on heterogeneous surfaces through multilayer adsorption,(27) several authors reach the same conclusion and are based on the fact that this equation is empirical therefore it was taken for heterogeneous adsorptions.(28) It is represented by the following equation:

Where: qe is the adsorption capacity of the solute (mg adto/ g adte), k F is the Freundlich adsorption constant at unit concentration, n (dimensionless) is the adsorption intensity, and Ce is the concentration at equilibrium (mg/L), this equation is solved by nonlinear regression analysis.(27) Some characteristics of this model are listed in table 3.
Temkin isotherm
The Temkin isotherm corresponds to an adsorption process in the multilayer, including a factor where the interaction between adsorbent and adsorbate is considered. It is a suitable model to anticipate gas-phase adsorption. It is represented by the following equation:

Where:
R is the gas constant, T is the temperature, bT is related to the heat of sorption (J/mol), KT is Temkin’s constant, and Ce is the concentration at equilibrium. Some characteristics of this model are listed in table 3.
|
Table 3. Characteristics of the isotherm models |
|
|
Model |
Characteristics |
|
Langmuir |
It is the most widely used in adsorption and was obtained by studying kinetic assumptions, thermodynamics and statistical analysis.(19) |
|
Adsorbate binds to a single surface site until adsorbed.(19) |
|
|
An adsorbate molecule is attached to a single surface site.(19) |
|
|
With increasing distance from the surface adsorbate, the frequency of intermolecular attractive forces such as van der Waals forces, ion-ion forces, and hydrogen bridges, decreases (19) |
|
|
Freunlich |
The Freundlich model has been considered an empirical equation with no specific physical meaning(27) Adsorption can occur chemically or physically.(19) |
|
It is used to represent the nonlinear adsorption phenomenon.(27) |
|
|
Temkin |
It is characterized by uniform energy distribution until the maximum binding energy is reached.(29) |
|
|
This model hypothesizes in which the decrease in adsorption enthalpy causes the degree of coating on the adsorbent to increase.(29) |
Adsorption thermodynamic parameters
Enthalpy is a thermodynamic function that provides information of the energy in a system, it is fundamentally important for geochemistry because of its relation to heat capacity, its role in defining and giving the temperature dependence of free energy and its relation to the entropy of the system environment.
Entropy provides the criteria for predicting the direction of spontaneous natural processes and the equilibrium state of a system. Entropy is a state function, meaning that its value is determined by the state of a system and is independent of the process.
The standard enthalpy and standard enthalpy change can be calculated by means of the linear Van’t Hoff equation according to the First and Second thermodynamic laws combined by means of the following equation:

A positive value of ΔH° shows that adsorption is endothermic, conversely, negative values mean that the process occurs exothermically.(19)
A standard state in thermodynamics represents temperature and pressure values of 298K and 1 atm, respectively, in this standard phase the formation energy for an element is zero in the normal state.
To find the spontaneity values of the processes the following equation is taken into account:
![]()
Where R is the gas constant, T the temperature, and K0 indicates the distribution coefficient and is given by K0 = qe/ce. For negative ΔG° values the adsorption process occurs spontaneously.(19)
Adsorption of ions on aqueous solutions of lanthanum, cerium and europium on different organic adsorbents.
Adsorption of lanthanum
Gallardo et al. studied the adsorption of La(III) in aqueous solutions through a process carried out at 20 °C, evaluating walnut shells (WS) as a bioadsorbent. To characterize WS before and after REE adsorption, FTIR spectroscopy was used to identify functional groups, SEM provided information on the material’s surface, and thermogravimetry was employed to determine the mass present before and after adsorption. The acid pH values were adjusted in batch experiments until the optimum adsorption condition was found to be pH 5,5, the isotherm models compared in this study were Freundlich, Langmuir and Temkin, all the isotherms showed a high correlation, but the one taken into account was Freundlich with a higher adjustment.(30)
Kusrini et al.(31) carried out studies of adsorption of La in aqueous solutions starting from a bioadsorbent that comes from agricultural waste, such as the inner part of the durian bark (Durio zibethinus), obtained important parameters in adsorption as the equilibrium time, pH, and the appropriate temperature for adsorption, pH, and the appropriate temperature for the elimination of this ion, resulting in an adsorption capacity of 71 mg of lanthanum per gram of bioadsorbent, for which it is convenient to mention that it would be a viable alternative in the field of wastewater contaminated with metal ions. During the preparation of the adsorbent, the external crust was removed, and the internal material was crushed into pieces approximately 1 cm in size. This was followed by a drying process, after which the material was milled to obtain particles of approximately 500 micrometers.
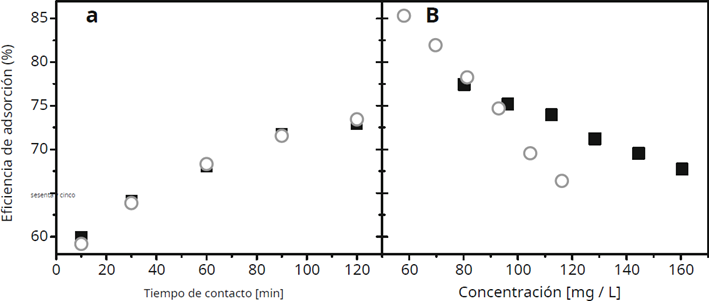
Figure 4. The black box refers to La ion, a) adsorption efficiency vs contact time, b) adsorption efficiency vs initial concentration(31)
Approximately 500 micrometers. When evaluating the isotherm models, Langmuir and Freundlich were considered, and the model with the best correlation was Langmuir. Figure 4 shows the graphs relating the adsorption capacity to the contact time and the initial concentration. Analyzing the thermodynamic parameters, they concluded that the process is reported to be exothermic.
The longer the contact time, the higher the percentage of La adsorption. On the other hand, the higher the initial concentration, the lower the adsorption capacity decreases. Increasing the La ion concentration limits the interaction between metal ions and the adsorbent due to the saturation of the adsorbent’s active sites. Therefore, the optimal adsorption depends on the ratio of metal ion concentration to adsorbent dosage.(34)
Zhao et al.(32) studied the adsorption of La (lll) using a biochar adsorbent derived from wheat straw as an effective and economical material in the recovery of lanthanides, the experiment was carried out in batch in a discontinuous process by adding different amounts of biochar, the pH studied was 5 having acidic characteristics, Finally, two empirical models of adsorption isotherms, Langmuir, Freundlich, were analyzed at three different temperatures at 20, 40 and 60ºC, finding that the adsorption of the La (lll) removal process increased with temperature, the model that best fit was the Langmuir model. Concerning Thermodynamic parameters were calculated, including ΔGº, ΔHº, and ΔSº, indicating that the process is endothermic and spontaneous.
In turn, Kusrini et al.(33) Conducted studies in 2018 using pectin bioadsorbent extracted from durian peel, the adsorption process was carried out discontinuously where the pH, the amount of pectin, the contact time and the appropriate temperature for adsorption were studied, Figure 5 shows graphically the percentage of adsorption concerning the dependence of the contact time and the initial concentration, the pH that was adequate for the adsorption process was found to be 4, with an adsorption equilibrium time of 90 minutes and different tests were carried out with temperatures of 25, 30, 40, 50 and 60 ° C, where it was found that the best adsorption was at 25°C. Subsequently, the Freundlich and Temkin isotherms were studied, as the process resulted in a heterogeneous and multilayer formation, allowing La ions to diffuse and penetrate the different layers through the pores on the surface of the pectin particles. On the other hand, the thermodynamics of lanthanum adsorption on pectin was studied, resulting in an exothermic and spontaneous process.
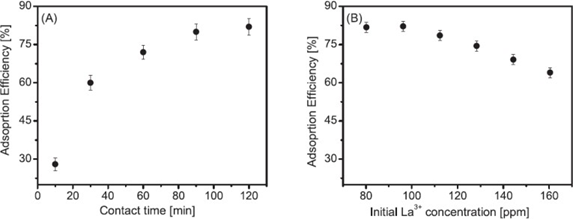
Figure 5. Adsorption efficiency vs A) contact time, B) initial concentration(33)
The adsorption percentage increased with the passage of time increasing in a non-linear way until reaching equilibrium, this indicated that the La ions occupied the active sites of the pectin, as time passed the sites were filled and passed to a saturation state at equilibrium, on the other hand, at an initial concentration of 80 ppm the efficiency reached the maximum
adsorption capacity of approximately 80 % and decreased with increasing initial concentration.
Kosheleva A et al.(34) studied the adsorption of La with a microalgae residual biomass adsorbent (Chlorella sorokiniana) using chitosan and millet husk. Chitosan was extracted from by-products of industrial processing of crustaceans and crab shells. The experiments were carried out in batches where the amount of adsorbent used was 0,1 g, the pH studied was 1,54 and 4,24, the whole adsorption process was handled at room temperature (20°C) and with a contact time of 60 min, where it later reached equilibrium and the adsorption isotherms were studied using the Langmuir and Freundlich model, as a result the model that best adjusted was the Langmuir model. This bioadsorbent was found to be viable in the adsorption of La, with metal ion removal exceeding 80 %.
A summary of the most important adsorption parameters reported in the literature for various adsorbents is presented in table 4. The values of the isotherm equations reported by the different authors can be found in table 4.
|
Table 4. Compilation of data obtained in the study of adsorption of lanthanum ions in aqueous solutions |
|||||||||
|
Adsorbent |
Lanthanum ion |
pH |
Equilibrium time (min) |
Temperature |
Amount of adsorbent (g) |
Isotherms |
qe (mg/g) |
C0 (mg / L) |
Reference |
|
Nutshell. |
La (lll) |
5,5 |
720 |
20ºC. |
4x10-6 |
Freundlich |
7,16 |
Data not reported. |
(30) |
|
Durian bark. |
La (lll) |
5 |
120 |
30ºC |
0,25 |
Langmuir |
80,3 |
80 |
(31) |
|
Biochar derived from straw. |
The (lll) |
5 |
75 |
20 ºC |
Not reported. |
Langmuir |
80 |
30 |
(32) |
|
Pectin extracted from durian peel. |
The (lll) |
4 |
90 |
25°C |
0,25 |
Freundlich , Temkin. |
41,2 |
80 |
(33) |
|
Residual microalgae biomass with chitosan and millet husk |
The (lll) |
4,24 |
60 |
20ºC. |
0,1 |
Langmuir, Freundlich |
34,53 |
100 |
(34) |
Cerium adsorption
One of the organic adsorbents used for the adsorption of Ce(IV) is activated carbon developed from rice straw. This ecological alternative aids in the adsorption of cerium ions in aqueous solutions. The experiments were conducted in a batch system, where the evaluation included pH, equilibrium time, optimum temperature, amount of adsorbent, kinetic, and isotherm models. The maximum adsorption capacity was found at a pH value of 4, with an adsorption equilibrium time of 500 minutes. Temperature is a significant factor influencing the adsorption of metal ions; in this study, samples were taken at different temperatures of 20, 25, 30, 35, and 40°C. It was found that the optimum temperature range for the highest adsorption of cerium is from 35°C to 40°C, using an adsorbent base of 0,02 g. The adsorption isotherms of cerium ions were determined in the laboratory through batch tests, based on experimental data obtained using Langmuir, Freundlich, and Temkin adsorption models. The adsorption of cerium by activated carbon also depends on the initial concentration of ions, in figure 7 this relationship is evident, the amount of cerium adsorbed on activated carbon increased directly with the initial concentration of metal ions until finding the abrupt change in the range of 200 to 250 (mg/L) where a steady state is evident where the amount of adsorption does not improve due to increasing concentration.(35)
The adsorption model that yielded the best fitting results was the Langmuir model, which provided a linear representation, as shown in figure 6.

Figure 6. Linear representation Langmuir model(35)
A novel bioadsorbent derived from cyanobacteria of the genus Arthrospira, commonly known as spirulina, was studied. This material was collected from water ponds in Israel and cultivated for a few months in an Erlenmeyer flask. The study was conducted to investigate the effects of contact time, pH, temperature, and adsorbent amount on achieving adequate cerium (III) adsorption. The isotherms used in this study were Langmuir and Freundlich; both were adjusted in the best way, obtaining high correlation values. In short, it can be deduced from the isotherm fit that REE bioadsorption exhibits an intermediate behavior between mono- and multilayer adsorption mechanisms.

Figure 7. Effects of initial cerium ion concentration on cerium adsorption by activated carbon(35)
Akbas YA et al.(36) performed the study of Ce adsorption in aqueous solutions by magnetic olive pomace modified with phosphoric acid (H₃PO₄) and iron chloride (FeCl₃), this pomace was collected after olive oil production, the biomaterial was characterized by SEM where the morphology of the sample was analyzed, in figure 8 was presented the SEM analysis of magnetite-olive pomace nanocomposite that when modified evidenced the presence of Fe nanoparticles in different sizes, where the adhesion of iron chloride on the surface is satisfactorily realized of the pomace. Subsequently, important factors such as pH, contact time, temperature, and the optimum amount of adsorbent for Ce adsorption were studied. These values are recorded in table 5. Concerning the isotherms, the Freundlich and Langmuir models were considered, and it was found that the Langmuir model best adjusted to the process conditions. Finally, thermodynamics was studied, and it was concluded that the system occurred endothermically and non-spontaneously.

Figure 8. Analysis of magnetite-olive pomace nanocomposite
Gao, S. et al.(37) studied in 2018 the adsorption of Ce using sawdust modified with polyacrylic acid, this material was chosen because it is a low-cost waste, the modification was made in order to improve the adsorption capacity to metal ions due to the presence of ion exchange groups, Isothermal, thermodynamic and kinetic studies were carried out in order to identify the pH, the contact time and the optimum temperature at which the experiment yielded better results, the pH was studied in a range of 1-7 where finally it was found that the best was 6. On the other hand, the Langmuir and Freundlich isotherms were studied. To conclude, thermodynamics was studied and concluded to have an endothermic and spontaneous behavior.
Below, in table 5, is a summary of the most important adsorption parameters reported in the literature for various adsorbents. The values of the isotherm equations reported by the different authors can be found in table 5.
|
Table 5. Compilation of data obtained in the study of adsorption of Cerium ions in aqueous solutions |
|||||||||
|
Adsorbent |
Cerium ion |
pH |
Equilibrium time (min) |
Temperature |
Amount of adsorbent (g) |
Isotherm |
qe (mg/g) |
C0 (mg / L) |
Reference |
|
Activated carbon a from rice straw. |
cerium (lV) |
4 |
500 |
35ºC. |
0,02 |
Langmuir |
4,13 |
50-300 |
(35) |
|
Spirulina biomass. |
cerium (lll) |
5- 5,5 |
300 |
27°C |
0,1 |
Langmuir, Freundlich |
7,2 |
10 |
(39) |
|
Pomaceolive pomace magnetic. |
cerium (lll) |
6 |
45 |
40°C |
0,04 |
Langmuir |
90,90 |
100 |
(35). |
|
Modified sawdust. |
cerium (lll) |
6 |
120 |
30°C |
0,02 |
Langmuir, Freundlich |
153,90 |
200 |
(36) |
Adsorption of europium
The adsorption of Eu(III) was performed using a new environmentally friendly material, namely cellulose functionalized with thiourea. Cellulose is the most abundant biomolecule. It is part of the terrestrial biomass, the functionalized adsorbent material was characterized by using FTIR, The influence of the adsorption parameters (adsorbent dose, time, temperature and initial metal concentration) on the adsorption capacity was investigated, giving as results, a maximum adsorption capacity in the 30 minutes of the process and a temperature of 24,8ºC. Thermodynamic studies were conducted to determine whether the adsorption processes under investigation are spontaneous or not, yielding a spontaneous and endothermic process. Finally, the experimental data were fitted using two different nonlinear isotherm models: Langmuir and Freundlich. The Langmuir model yielded better results.(38)
Gallardo et al.(30) studied the adsorption of Eu(III) in aqueous solutions using walnut shell (WS) to determine whether this bioadsorbent can efficiently adsorb various rare earth elements. FTIR spectroscopy, SEM, and thermogravimetry were used to characterize WS before and after REE adsorption. The pH values were adjusted in batch experiments until
The isotherm models compared in this study were those of Freundlich, Langmuir, and Temkin. All the isotherms obtained good results, but the one that was most adjusted and taken into account was Langmuir. The entire process was conducted at a temperature of 20°C.
Kosheleva A et al.(35) performed an experiment on Eu adsorption based on a microalgae residual biomass adsorbent (Chlorella sorokiniana) plus chitosan, which was extracted from by-products of industrial processing of crustaceans and crab shells. The experiments were carried out in batches where the amount of adsorbent used was 0,1 g, the pH studied was 1,54 and 4,24, the whole adsorption process was handled at room temperature (20°C) and with a contact time of 60 min, where it later reached equilibrium where the adsorption isotherms were studied using the Langmuir and Freundlich model, as a result the model that best adjusted was that of Langmuir. This bioadsorbent was viable in Eu adsorption, with metal ion removal exceeding 80.
Lapo et al.(23) studied in 2020 the performance of a new adsorbent from banana waste by analyzing three types of adsorbents, banana rachis (BR), banana stalk (BPS) and banana peel (BP), all three gave excellent results, but it was emphasized that BR adsorbed more amount of Eu, the experiment was carried out by varying the concentration of Eu in ranges of 10-300 mg/L. The influence of pH (2–5) was observed. In figure 9, the dependence of pH on the amount of adsorption can be observed. The contact time, kinetics, and isotherm models were analyzed. The nonlinear isotherms studied were Langmuir and Freundlich; the model that fitted best was the Langmuir model. Regarding the kinetic models, the best-fitting model was the pseudo-second-order model. Regarding the characterization of the material, which was analyzed using FTIR and SEM, the morphology of BR presents a simultaneously rough and homogeneous structure. In conclusion, this adsorbent belongs to the group of those with the most adsorption capacity based on the presence of carboxylic groups.

Figure 9. pH dependence with Eu adsorption
Shen et al. conducted experiments on Eu adsorption using a Serratia marcescens adsorbent, a Gram-negative bacillus from the Enterobacteriaceae family. This bacillus was isolated in the laboratory, where the same authors carried out the entire adsorption process. The parameters directly related to the adsorption capacity, such as pH, contact time, kinetic, and isothermal parameters, were analyzed. The results obtained determined that, kinetically, the model that best fitted was the pseudo-second order model. In Figure 10, the behavior between the two kinetic models can be observed. Adsorption was controlled by chemisorption, with the Langmuir isotherm being the most suitable option for the process. The adsorption capacity also depends on the initial concentration of Eu. In figure 11, it is evident that from the increase of the concentration, good results were obtained until it reached 30 mg /L, and the adsorption remained constant because the adsorption sites in the biosorbent were saturated with Eu.(39)

Figure 10. Kinetic analysis of Eu adsorption with Serratia marcescens(39)

Figure 11. Initial Eu concentration vs adsorption capacity(39)
If we make a comparison of figures 10 and 11 we find the adsorption capacity concerning the initial concentration of Ce and Eu ions, analyzing these figures it can be inferred that the adsorption of ions is highly dependent on the concentration so it is one of the important parameters to take into account in the adsorption process, most processes are performed in a specific range of C0, for Ce is not an exception and involves experiments between 50-300 (mg/L) in ions of this element, the adsorption capacity increases to the degree that a higher concentration of ions is provided, but this is not relevant because, along the process, the adsorption reaches a limit where it remains constant. After all, the active sites of the adsorbent are saturated and causes the adsorption collapse, on the other hand, the adsorption capacity of Eu by Serratia marcescens starts with the initial concentration of 10 mg/L until reaching experiments with higher concentrations of 90 mg/L, where the same thing happens, adsorption is inefficient at higher concentration values so that the active sites are filled with adsorbed ions and does not facilitate adsorption, so increasing the concentration does not mean that the adsorption efficiency improves, but on the contrary it has a limit.
Table 6 below provides a summary of the most important Eu adsorption parameters reported in the literature for various adsorbents. The values of the isotherm equations reported by the different authors are shown in table 7.
|
Table 6. Compilation of data obtained in the study of adsorption of Europium ions in aqueous solutions |
|||||||||
|
Adsorbent |
Europium ion |
pH |
Equilibrium time (min) |
Temperature |
Amount of adsorbent (g) |
Isotherm |
qe (mg/g) |
C0 (mg / L) |
Reference |
|
Cellulose functionalized with thiourea. |
Eu (lll) |
- |
30 |
24,8ºC |
0,1 |
Langmuir |
27 |
50 |
(38) |
|
Nutshell. |
Eu (lll) |
4 |
720 |
20°C. |
- |
Langmuir |
6,92 |
Data not reported. |
(30) |
|
Residual microalgae biomass plus chitosan. |
Eu (lll) |
4,24 |
60 |
20ºC. |
0,1 |
Langmuir, Freundlich . |
31,91 |
10 |
(34) |
|
Banana rachis. |
Eu (lll) |
4,5 |
50 |
20°C. |
0,025 |
Langmuir |
100 |
100 |
(23) |
|
Serratia marcescens. |
Eu (lll) |
5 |
120 |
25°C |
0,3 |
Langmuir |
115,36 |
30 |
(39) |
The pH value is one of the most important parameters for studying adsorption in aqueous solutions, as its effect on the surface charge of the adsorbent, the degree of ionization, the adsorbate species, and the functional groups on the surface all influence the process.(36) All experiments occupied a pH range as a reference belonging to the acidic scale, this was possible due to the dissociation of the carboxyl groups present in the studied material (biomass), as results at higher acid values the adsorption of metals was increasing, enabling more sites available for adsorption to occur and improving the removal efficiency.
The contact time was necessary to carry out the kinetic study of the process, together with the temperature and the amount of adsorbent, these have a significant influence on the efficiency in the adsorption of metal ions, the increase of the contact time and the temperature did not mean a high efficiency in the removal of the ions.
For the equilibrium study it is essential to use isotherm models to give us an idea of how the adsorption mechanism occurs, in the literature reviewed it was possible to note an excellent efficiency in the Langmuir and Freundlich models, being the nonlinear equations the ones that best fit the experiments, the summary of the parameters in the isotherm equations reported by the different authors is shown in table 7. Langmuir has a significant advantage because its mechanism occurs in a monolayer on a homogeneous surface, and its reaction belongs to chemisorption, which allows the adsorbate molecules to adsorb only on the adsorption sites of the adsorbent, thereby achieving the same activation energy for the molecules. On the other hand, Freundlich assumes his mechanism applies to multilayer and heterogeneous surfaces; the results of the equation’s parameters were obtained through nonlinear regression. Finally, a comparison is made between these two models to describe which one exhibits the higher adsorbent performance. Isotherms generally represent the equilibrium ratio between adsorbate adsorbed on the surface and the amount of adsorbate in solution.(34)
Among the studied adsorbents with higher adsorption capacity are biochar derived from wheat straw, magnetic olive pomace, serratia marcescens and modified sawdust, having significant advantages for future research in the adsorption of La, Ce and Eu ions, it could be said that the capacity of elimination and recovery using these bioadsorbents is optimal and helps us to have a broad idea of the occupation of biomass as an environmental alternative taking advantage of the fact that most of these materials can be found more easily. Their cost is not high; the study of the literature reveals that these types of adsorbents mostly follow a Langmuir isotherm model in the monolayer and are of a chemical nature, in other words, chemisorption, an irreversible process formed by covalent chemical bonds between the adsorbed molecules.
For the recovery of the bioadsorbents, a desorption and purification process was carried out, taking into account that most of the materials studied can be used for various tests, which means they are reusable materials that can reduce operating costs in future research.
|
Table 7. Summary of parameters in isotherm equations reported by different authors |
||||||||||||
|
Adsorbent |
Ion |
Langmuir model |
Freundlich model |
Temkin model |
Reference |
|||||||
|
|
|
qm (mg/g) |
bL (L/mg) |
R2 |
kf (L/mg) |
n |
R2 |
kT (kJ/ mol) |
bT |
R2 |
|
|
|
Bark of durian |
La (III) |
71 |
38,8 |
- |
- |
- |
- |
- |
- |
- |
(33) |
|
|
Biochar derived from wheat straw. |
La (III) |
242,51 71 |
0,0238 |
0,9895 |
- |
- |
- |
- |
- |
- |
(32) |
|
|
Residual microalgae biomass of microalgae with chitosan and millet husk. |
The (III) |
4,28 |
- |
0,999 |
- |
- |
- |
- |
- |
- |
(34) |
|
|
Activated carbon from rice straw. |
cerium (lV) |
5,12 |
0,012 |
0,913 |
- |
- |
- |
- |
- |
- |
(35) |
|
|
Pomace magnetic olive pomace. |
cerium (lll) |
93,984 |
0,0024 |
- |
- |
- |
- |
- |
- |
- |
(36) |
|
|
Cellulose functionalized with thiourea. |
Eu (lll) |
32,27 |
0,052 |
0,9914 |
- |
- |
- |
- |
- |
- |
(38) |
|
|
Nutshell. |
Eu (lll) |
5,689 |
0,214 |
0,999 |
- |
- |
- |
- |
- |
- |
(30) |
|
|
Residual biomass of microalgae plus chitosan. |
Eu (lll) |
1,74 |
- |
0,991 |
- |
- |
- |
- |
- |
- |
(34) |
|
|
Rachis of banana. |
Eu (lll) |
85,49 |
0,07 |
0,87 |
- |
- |
- |
- |
- |
- |
(23) |
|
|
Serratia marcescens. |
Eu (lll) |
116,30 38 |
0,2815 7 |
0,9947 7 |
- |
- |
- |
- |
- |
- |
(39) |
|
|
Modified sawdust. |
cerium (lll) |
155,38 |
1,2302 |
- |
71,9 666 |
5,2 092 |
- |
- |
- |
- |
(37) |
|
|
Spirulina biomass. |
cerium (lll) |
- |
- |
- |
1,26 |
2,5 1 |
- |
|
|
|
(40) |
|
|
Walnut shells |
La (III) |
- |
- |
- |
11,3 46 |
6,6 18 |
0,999 |
- |
- |
- |
(30) |
|
|
Pectin extracted from the durian peel |
The (III) |
- |
- |
- |
1,14 6 |
4,1 2 |
- |
0,387 |
3,268 |
- |
(31) |
|
A comparison of rare earth elements with a biosorbent derived from coffee husk for the efficient removal of toxic heavy metal ions, such as lead and cadmium, from wastewater reveals that the adsorption process also performs satisfactorily.
Converting to biosorbents through a practical approach to produce an efficient, value-added, and low-cost adsorbent, coffee waste is found in large quantities due to its unique structure and physicochemical characteristics. Graphically, the adsorbent process is described in figure 12.
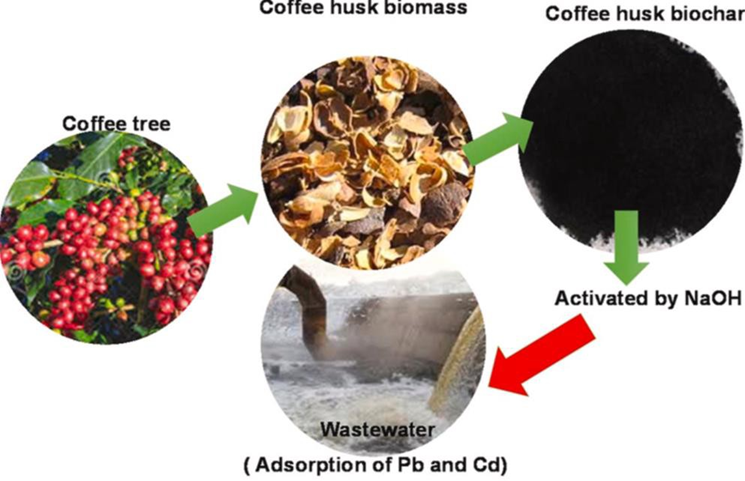
Figure 12. Process of obtaining the adsorbent(41)
They investigated the fundamental adsorption parameters such as pH, contact time, initial concentration, kinetic and isothermal models. The pH range studied was 2- 7 with a contact time of 90 minutes, as a result the study followed the Freundlich model and the second order kinetic model, with a maximum adsorption capacity of Cd of 116,3 (mg / g) and Pb 139,5 (mg /g), finally it was proved that the material could be reused and contributes efficiently in the adsorption of heavy metal ions in water decontamination.
Finally, a comparison of the isotherm models studied by Quyen et al.(41) is presented in table 8. It is shown that Freundlich is the model most suitable for the removal of heavy metals.
|
Table 8. Isotherm parameters |
||||||
|
Heavy metals |
Langmuir model |
Freundlich model |
||||
|
qm |
bL |
R2 |
Kf |
n |
R2 |
|
|
Cd2+ |
116,3 |
0,26 |
0,82 |
0,51 |
0,34 |
0,95 |
|
Pb2+ |
139,5 |
0,18 |
0,86 |
0,41 |
0,58 |
0,97 |
In the kinetic and activation energy study of Eu adsorption by thiourea functionalized cellulose it was evidenced that the temperature also influences the maximum adsorption capacity of the modified cellulose so it was concluded that the Eu ion adsorption processes were best described by the pseudo-second order kinetic model, later with the found rate constant they performed the Arrhenius plot to find the value of the activation energy, Negrea et al.(38) found that the adsorption of Eu ions needs an activation energy of 2,99 x10-3 kJ /mol. figure 13 represents the Arrhenius plot reported by the authors of that research.(42,43)

Figure 13. Arrhenius plot for Eu adsorption by functionalized cellulose
The thermodynamic parameters and kinetic models analyzed from the few authors who presented this information in their respective adsorption studies are shown in table 9. This study is not carried out in most of the articles, due to its complexity and exhaustive analysis.(44,45)
|
Table 9. List of thermodynamic parameters and kinetic models for REE adsorption on different adsorbents |
|||||||
|
Adsorbent |
REE |
Temperature ºC |
Δ H ° (kJ / mol) |
Δ S ° (J / mol ∙ K) |
Δ G°(kJ / mol) |
Kinetics |
Reference |
|
Cellulose functionalized with thiourea. |
Eu (lll) |
25 |
1,87 |
8,33 |
-0,608 |
pseudo- second order |
(38) |
|
Biochar derived from straw. |
The (lll) |
20 |
35,39 |
104,71 |
-40,04 |
pseudo-second order. |
(32) |
|
Pectin extracted from durian peel. |
The (lll) |
25 |
0,012 |
0,02 |
-6,2 |
pseudo- second order. |
(31) |
|
Magnetic olive pomace |
Cerium (lll) |
40 |
7,4495 |
0,0081 |
4,9081 |
pseudo-second order. |
(36) |
|
Sawdust modified |
cerium (lll) |
30 |
16,66 |
65,35 |
- 3,12 |
pseudo- second order. |
(37) |
Thermodynamic studies are performed in order to evaluate the spontaneity of the process, for this the change in Gibbs energy is calculated where we can analyze that at negative values it occurs spontaneously, at positive values the process occurs non-spontaneously in the sense that there will be no formation in products, when the value is zero in the change of Gibbs free energy it is said that the system is in equilibrium state and both reactants and products remain constant.
Analyzing table 9. In the Gibbs free energy values, it can be seen that all adsorbents, except magnetic olive pomace, occur spontaneously. The standard enthalpy change provided us with a means to evaluate whether the process absorbed or released energy. In all the literature, it was observed that the process was endothermic, with heat being transferred from the surroundings to the system.
Transfer of heat from the surroundings to the system. The entropy value determined the disorder of the system; if it was positive, it indicated a higher degree of disorder. The adsorbent that obtained the least disorder in the system was the magnetic olive pomace.
CONCLUSIONS
The literature review conducted revealed that adsorption is one of the most promising methods due to its high efficiency and low operational costs for removing Lanthanum, Cerium, and Europium from aqueous solutions, highlighting the importance of evaluating variables such as contact time, pH, and temperature.
The removal of La and Eu based on the compilation of information by Gallardo et al.(33) it was found that at a pH of 4, the adsorption was 87 % for Eu and 85 % for La. They report that this occurred due to the high carbon content of the walnut shell, which served as the adsorbent.
The adsorption of La by durian bark adsorbent reached its maximum capacity at a pH of 4, with a 72,8 % removal.
Sadovsky et al.(40) reported that the amount of Ce adsorption at pH 5 was 90 %. For the recovery of the adsorbent, three adsorption-desorption cycles were performed, resulting in a recovery rate of 97 % for the spirulina biomass. This relates to its good utility as a bioadsorbent and feasibility for the recovery of Ce ions.
The bioadsorbent of microalgae residual biomass, combined with chitosan and millet husk, for La removal at pH 4 resulted in more than 80 % adsorption.(34)
In general, the adsorption capacity of bioadsorbents was very effective, and it can be concluded that the removal capacity for most of the La, Ce, and Eu ions was more than 70 %.
In the kinetic studies, modeling was performed to determine which step controlled the removal rate, this could be external mass transfer, internal diffusion or adsorption; in most of the literature, the kinetics was controlled in the adsorption step so it was possible to identify that the pseudo-second order model provided a better fit, leading to the conclusion that most of the experiments that were carried out were governed by Quimi sorption phenomena.
For modeling the adsorption isotherms, the literature study revealed that the Langmuir and Freundlich models fitted the processes best, with R² values of more than 0,9. The former was characterized by adsorption in a monolayer with homogeneous surfaces, while the latter was characterized by adsorptions occurring in multilayers and heterogeneously. The adsorbents that the Langmuir model governed are durian bark, biochar derived from wheat straw, microalgae residual biomass with chitosan, millet husk, activated carbon from rice straw, magnetic olive pomace, thiourea-functionalized cellulose, walnut shell, microalgae residual biomass plus chitosan, banana rachis, and Serratia marcescens.
It was possible to evaluate the dependence of the thermodynamic parameters and their importance. Most of the literature studied showed that adsorption is a spontaneous and endothermic phenomenon, except for the olive pomace adsorbent, where the system absorbed thermal energy from its environment.
Thus, it can be concluded that the adsorption process using organic matter and activated carbon is an effective and novel alternative that demonstrates a high adsorption of La, Ce and Eu ions in aqueous solutions, which can be applied in the treatment of wastewater contaminated with rare earths and help in the recovery of these metals for environmental remediation.
BIBLIOGRAPHIC REFERENCES
1. Jaireth S, Hoatson DM, Miezitis Y. Geological setting and resources of the major rare-earth-element deposits in Australia. Ore Geol Rev. 2014;62:72-128. https://doi.org/10.1016/j.oregeorev.2014.02.008
2. Moldoveanu GA, Papangelakis VG. Recovery of rare earth elements adsorbed on clay minerals: I. Desorption mechanism. Hydrometallurgy. 2012;117-118:71-8. https://doi.org/10.1016/j.hydromet.2012.02.007
3. Anastopoulos I, Bhatnagar A, Lima EC. Adsorption of rare earth metals: A review of recent literature. J Mol Liq. 2016;221:954-62. https://doi.org/10.1016/j.molliq.2016.06.076
4. Binnemans K, Jones PT, Blanpain B, van Gerven T, Yang Y, Walton A, et al. Recycling of rare earths: A critical review. J Clean Prod. 2013;51:1-22. https://doi.org/10.1016/j.jclepro.2012.12.037
5. Liu S, Fan HR, Yang KF, Hu FF, Wang KY, Chen FK, et al. Mesoproterozoic and Paleozoic hydrothermal metasomatism in the giant Bayan Obo REE-Nb-Fe deposit. Precambrian Res. 2018;311:228-46. https://doi.org/10.1016/j.precamres.2018.04.021
6. Liu T, Chen J. Extraction and separation of heavy rare earth elements: A review. Sep Purif Technol. 2021;276:119263. https://doi.org/10.1016/j.seppur.2021.119263
7. Jowitt SM, Werner TT, Weng Z, Mudd GM. Recycling of the rare earth elements. Curr Opin Green Sustain Chem. 2018;13:1-7. https://doi.org/10.1016/j.cogsc.2018.02.008
8. Mancheri NA, Sprecher B, Bailey G, Ge J, Tukker A. Effect of Chinese policies on rare earth supply chain resilience. Resour Conserv Recycl. 2019;142:101-12. https://doi.org/10.1016/j.resconrec.2018.11.017
9. Dushyantha N, Batapola N, Ilankoon IMSK, Rohitha S, Premasiri R, Abeysinghe B, et al. The story of rare earth elements (REEs): Occurrences, global distribution, genesis, geology, mineralogy and global production. Ore Geol Rev. 2020;122:103521. https://doi.org/10.1016/j.oregeorev.2020.103521
10. Villela Olavarría D, Donoso Rojas F, Cantallopts Araya J. Situación actual del mercado de tierras raras y su potencial en Chile. 2016.
11. King H. REE - Rare Earth Elements and their Uses. 2017.
12. Willbold M. Europium. In: White WM, editor. Encyclopedia of Geochemistry: A Comprehensive Reference Source on the Chemistry of the Earth. Springer International Publishing; 2018. p. 462-4. https://doi.org/10.1007/978-3-319-39312-4_99
13. Chauvel C. Cerium. In: White WM, editor. Encyclopedia of Geochemistry: A Comprehensive Reference Source on the Chemistry of the Earth. Springer International Publishing; 2018. p. 226-9. https://doi.org/10.1007/978-3-319-39312-4_88
14. Chauvel C. Encyclopedia of Geochemistry. In: White WM, editor. Springer International Publishing; 2018. https://doi.org/10.1007/978-3-319-39312-4
15. Mozaffari Majd M, Kordzadeh-Kermani V, Ghalandari V, Askari A, Sillanpää M. Adsorption isotherm models: A comprehensive and systematic review (2010−2020). Sci Total Environ. 2021;151334. https://doi.org/10.1016/j.scitotenv.2021.151334
16. Zhang D, Qu R, Zhang H, Zhang F. Differentiation of chemisorption and physisorption of carbon dioxide on imidazolium-type poly(ionic liquid) brushes. J Wuhan Univ Technol Mater Sci Ed. 2020;35(4):750-7. https://doi.org/10.1007/s11595-020-2317-2
17. Wang J, Guo X. Adsorption isotherm models: Classification, physical meaning, application and solving method. Chemosphere. 2020;258:127279. https://doi.org/10.1016/j.chemosphere.2020.127279
18. Niño Arias IV, Ortíz Ramírez D. Evaluación de dos clases de carbón activado granular para su aplicación efectiva en la remoción de fenoles en los vertimientos de una empresa de jabones. Bogotá: Universidad de La Salle; 2008. https://ciencia.lasalle.edu.co/ing_ambiental_sanitaria
19. Paul Nayagam JO, Prasanna K. Utilization of shell-based agricultural waste adsorbents for removing dyes: A review. Chemosphere. 2021;132737. https://doi.org/10.1016/j.chemosphere.2021.132737
20. Tran HN, Lima EC, Juang RS, Bollinger JC, Chao HP. Thermodynamic parameters of liquid-phase adsorption process calculated from different equilibrium constants related to adsorption isotherms: A comparison study. J Environ Chem Eng. 2021;9(6):106674. https://doi.org/10.1016/j.jece.2021.106674
21. Geankoplis CJ. Procesos de transporte y operaciones unitarias.
22. Can N, Ömür BC, Altındal A. Modeling of heavy metal ion adsorption isotherms onto metallophthalocyanine film. Sens Actuators B Chem. 2016;237:953-61. https://doi.org/10.1016/j.snb.2016.07.026
23. Lapo B, Bou JJ, Hoyo J, Carrillo M, Peña K, Tzanov T, et al. A potential lignocellulosic biomass based on banana waste for critical rare earths recovery from aqueous solutions. Environ Pollut. 2020;264. https://doi.org/10.1016/j.envpol.2020.114409
24. Largitte L, Pasquier R. A review of the kinetics adsorption models and their application to the adsorption of lead by an activated carbon. Chem Eng Res Des. 2016;109:495-504. https://doi.org/10.1016/j.cherd.2016.02.004
25. Lapo B, Demey H, Carchi T, Sastre AM. Antimony removal from water by a chitosan-iron(III) biocomposite. Polymers. 2019;11(2). https://doi.org/10.3390/polym11020351
26. Chen Y, Tang J, Wang S, Zhang L. High selectivity and reusability of coordination polymer adsorbents: Synthesis, adsorption properties and activation energy. Microporous Mesoporous Mater. 2021;324:111309. https://doi.org/10.1016/j.micromeso.2021.111309
27. Mozaffari Majd M, Kordzadeh-Kermani V, Ghalandari V, Askari A, Sillanpää M. Adsorption isotherm models: A comprehensive and systematic review (2010−2020). Sci Total Environ. 2021;151334. https://doi.org/10.1016/j.scitotenv.2021.151334
28. Zaheer Z, Al-Asfar A, Aazam ES. Adsorption of methyl red on biogenic Ag@Fe nanocomposite adsorbent: Isotherms, kinetics and mechanisms. J Mol Liq. 2019;283:287-98. https://doi.org/10.1016/j.molliq.2019.03.030
29. Arroyo Ramírez LD-RRDM. Determinación de la cinética de adsorción de cloruros de vertimientos del sector agrícola cultivos energéticos, sobre carbón activado comercial. 2018.
30. Gallardo K, Castillo R, Mancilla N, Remonsellez F. Biosorption of Rare-Earth Elements From Aqueous Solutions Using Walnut Shell. Front Chem Eng. 2020;2. https://doi.org/10.3389/fceng.2020.00004
31. Kusrini E, Usman A, Sani FA, Wilson LD, Abdullah MAA. Simultaneous adsorption of lanthanum and yttrium from aqueous solution by durian rind biosorbent. Environ Monit Assess. 2019;191(8). https://doi.org/10.1007/s10661-019-7634-6
32. Zhao Q, Wang Y, Xu Z, Yu Z. The potential use of straw-derived biochar as the adsorbent for La(III) and Nd(III) removal in aqueous solutions. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-021-13988-2
33. Kusrini E, Wicaksono W, Gunawan C, Daud NZA, Usman A. Kinetics, mechanism, and thermodynamics of lanthanum adsorption on pectin extracted from durian rind. J Environ Chem Eng. 2018;6(5):6580-8. https://doi.org/10.1016/j.jece.2018.10.018
34. Kosheleva A, Atamaniuk I, Politaeva N, Kuchta K. Adsorption of rare earth elements using bio-based sorbents. MATEC Web Conf. 2018;245. https://doi.org/10.1051/matecconf/201824518001
35. Farahmand E. Adsorption of Cerium (IV) from Aqueous Solutions Using Activated Carbon Developed from Rice Straw. Open J Geol. 2016;6(3):189-200. https://doi.org/10.4236/ojg.2016.63017
36. Akbas YA, Yusan S, Sert S, Aytas S. Sorption of Ce(III) on magnetic/olive pomace nanocomposite: isotherm, kinetic and thermodynamic studies. Environ Sci Pollut Res Int. 2021. https://doi.org/10.1007/s11356-021-14662-3
37. Gao S, Luo T, Zhou Q, Luo W. A novel and efficient method on the recovery of nanosized CeO2 in Ce3+ wastewater remediation using modified sawdust as adsorbent. J Colloid Interface Sci. 2018;512:629-37. https://doi.org/10.1016/j.jcis.2017.09.032
38. Negrea A, Gabor A, Davidescu CM, Ciopec M, Negrea P, Duteanu N, et al. Rare Earth Elements Removal from Water Using Natural Polymers. Sci Rep. 2018;8(1). https://doi.org/10.1038/s41598-017-18623-0
39. Shen J, Liang C, Zhong J, Xiao M, Zhou J, Liu J, et al. Adsorption behavior and mechanism of Serratia marcescens for Eu(III) in rare earth wastewater. Environ Sci Pollut Res. 2021. https://doi.org/10.1007/s11356-021-14668-x
40. Sadovsky D, Brenner A, Astrachan B, Asaf B, Gonen R. Biosorption potential of cerium ions using Spirulina biomass. J Rare Earths. 2016;34(6):644-52. https://doi.org/10.1016/S1002-0721(16)60074-1
41. Quyen VT, Pham TH, Kim J, Thanh DM, Thang PQ, van Le Q, et al. Biosorbent derived from coffee husk for efficient removal of toxic heavy metals from wastewater. Chemosphere. 2021;284. https://doi.org/10.1016/j.chemosphere.2021.131312
42. Bharathi KS, Ramesh ST. Removal of dyes using agricultural waste as low-cost adsorbents: a review. Appl Water Sci. 2013;3(4):773-90. https://doi.org/10.1007/s13201-013-0117-y
43. Moses CO. Enthalpy and Entropy. In: White WM, editor. Encyclopedia of Geochemistry. Springer International Publishing; 2018. https://doi.org/10.1007/978-3-319-39312-4
44. Charnley SB. Chemisorption. In: Gargaud M, Amils R, Cernicharo Quintanilla J, Cleaves HJ, Irvine WM, Pinti DL, et al., editors. Encyclopedia of Astrobiology. Berlin, Heidelberg: Springer; 2015. https://doi.org/10.1007/978-3-662-44185-5_270
45. Zhu Y, Zheng Y, Wang A. A simple approach to fabricate granular adsorbent for adsorption of rare elements. Int J Biol Macromol. 2015;72:410-20. https://doi.org/10.1016/j.ijbiomac.2014.08.039
FINANCING
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORSHIP CONTRIBUTION
Conceptualization: Roxana Alejandra Ramirez Moriano, Jacqueline Corredor Acuña.
Data curation: Roxana Alejandra Ramirez Moriano, Jacqueline Corredor Acuña.
Formal analysis: Roxana Alejandra Ramirez Moriano, Jacqueline Corredor Acuña.
Research: Roxana Alejandra Ramirez Moriano, Jacqueline Corredor Acuña.
Methodology: Roxana Alejandra Ramirez Moriano, Jacqueline Corredor Acuña.
Project Management: Roxana Alejandra Ramirez Moriano, Jacqueline Corredor Acuña.
Resources: Roxana Alejandra Ramirez Moriano, Jacqueline Corredor Acuña.
Software: Roxana Alejandra Ramirez Moriano, Jacqueline Corredor Acuña.
Supervision: Roxana Alejandra Ramirez Moriano, Jacqueline Corredor Acuña.
Validation: Roxana Alejandra Ramirez Moriano, Jacqueline Corredor Acuña.
Visualization: Roxana Alejandra Ramirez Moriano, Jacqueline Corredor Acuña.
Writing - original draft: Roxana Alejandra Ramirez Moriano, Jacqueline Corredor Acuña.
Writing - proofreading and editing: Roxana Alejandra Ramirez Moriano, Jacqueline Corredor Acuña.